13.2: Nomenclature, Ligands, and Classification
- Page ID
- 386277
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The nomenclature of coordination compounds was described in an earlier section (Section 9.2). Organometallic compounds are named using this same system, so it may be helpful to review before proceeding. Here, we will point out some of the symbols and terminology that are used heavily in naming organometallic complexes.
Hapticity
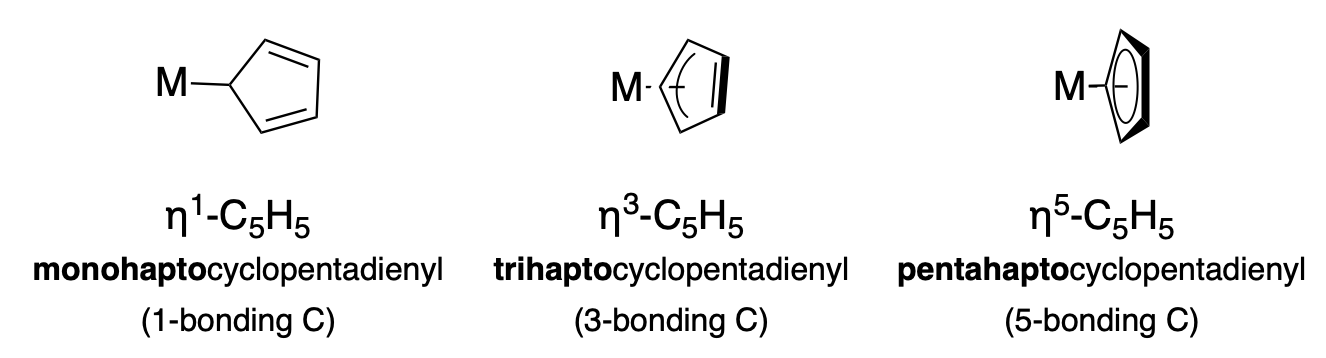
The eta (\(\eta\)) symbol is used to indicate the variable hapticity of ligands with conjugated \(\pi\) systems. For example, the cyclopentadiene anion (Cp, \(\ce{C_5H_5}\)) ligand has the capability to coordinate a metal ion in several ways due to its cyclic conjugated \(\pi\) system. The hapticity (the number of atoms involved in the metal bonding interaction) must be indicated in the formula with the \(\eta ^n\) notation, and in the name with the appropriate prefix. Figure \(\PageIndex{1}\) illustrates three different hapticities, and the relevant formula symbols and names.
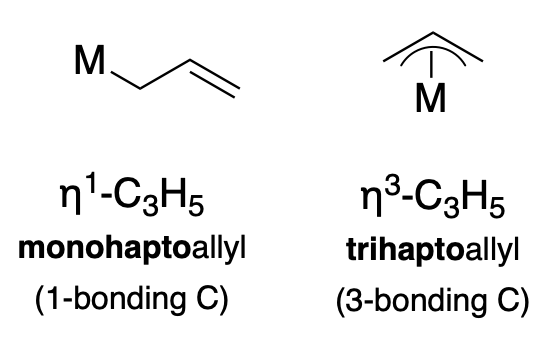
Another common ligand is the \(\pi\)-allyl anion (\(\ce{C_3H_3}\)), which can coordinate in either an \(\eta^1\) or \(\eta^3\) fashion, as illustrated below (Figure \(\PageIndex{2}\)).
Bridging ligands
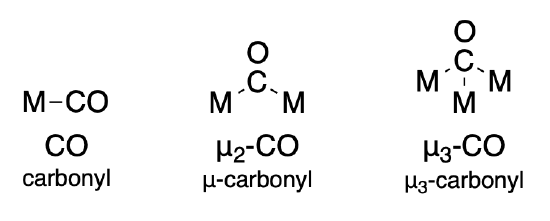
Ligands that bridge two or more metal ions are indicated using the symbol, mu (\(\mu\)). For a ligand that bridges \(n\) metal ions, the symbol is written as \(\mu _n\). However the subscript is often ommitted when \(n=2\). Some examples of the carbonyl ligand are given below. More specific rules for naming are found in Section 9.2.
Common Ligands and Classifications
Some common organic ligands found in organometallic compounds are listed below with their names and structures.
| Name | Structure | CBC description |
|---|---|---|
| Alkyl | 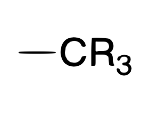 |
X |
| Carbonyl | 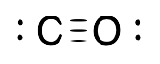 |
L |
| Carbene (alkylidene) | 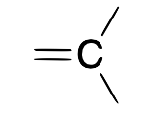 |
X2 |
| Carbyne (alkylidyne) | 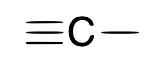 |
X3 |
| Ethylene | 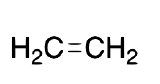 |
L |
| Acteylene | 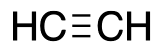 |
L |
| Acyl | 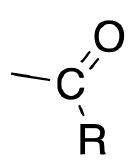 |
X |
| \(\pi\)-allyl (\(\ce{C_3H_5^-}\)) |  |
X for \(\eta^1\) LX for \(\eta^3\) |
| Cyclopropenyl (cyclo-\(\ce{C_3H_3}\)) |  |
X for \(\eta^1\) LX for \(\eta^3\) |
| Cyclobutadiene (cyclo-\(\ce{C_4H_4}\)) |  |
L2 for \(\eta^4\) |
| Cyclopentadienyl (cyclo-\(\ce{C_5H_5}\), Cp) | 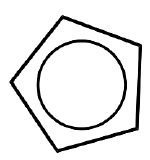 |
X for \(\eta^1\) LX for \(\eta^3\) L2X for \(\eta^5\) |
| Benzene | 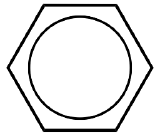 |
L3 for \(\eta^6\) |
| 1,5-cyclooctadiene (1,5-COD) |  |
L2 for \(\eta^4\) |
Covalent Bond Classification Method (CBC)
Later in this chapter, you will learn to count electrons. The way in which you count depends on what counting scheme you choose to use. But in any case, the way in which we "count" the electrons from ligands depends on the ligand type. The covalent bond classification method (CBC) has been in use since the late 1990's to classify ligands in organometallic complexes. This method classifies ligands into three main types as follows. (see Application of the Covalent Bond Classification Method for the Teaching of Inorganic Chemistry. Malcolm L. H. Green and Gerard Parkin, Journal of Chemical Education 2014 91 (6), 807-816, DOI: 10.1021/ed400504f.)
| CBC Ligand Class/Type | Examples | Use in neutral Ligand Method of Electron Counting | Use in Donor Pair Method of Electron Counting |
|---|---|---|---|
| X-type | \(\ce{H^-, Cl^-, H_3C^-}\) | Considered neutral Donates 1 electron to the metal |
Considered anionic Donates 2 electrons to the metal |
| L-type | \(\ce{CO, NH_3, H_2O}\) | Considered neutral Donates 2 electrons to the metal |
Considered neutral Donates 2 electrons to the metal |
| Z-type (rarely used) | \(\ce{BR_3}\), Lewis acids |
Considered neutral Accepts 2 electrons |
Considered cationic Donates 0 electrons |


