11.3.6: Tetrahedral Complexes
- Page ID
- 377933
Transitions in tetrahedral complexes are Laporte-allowed
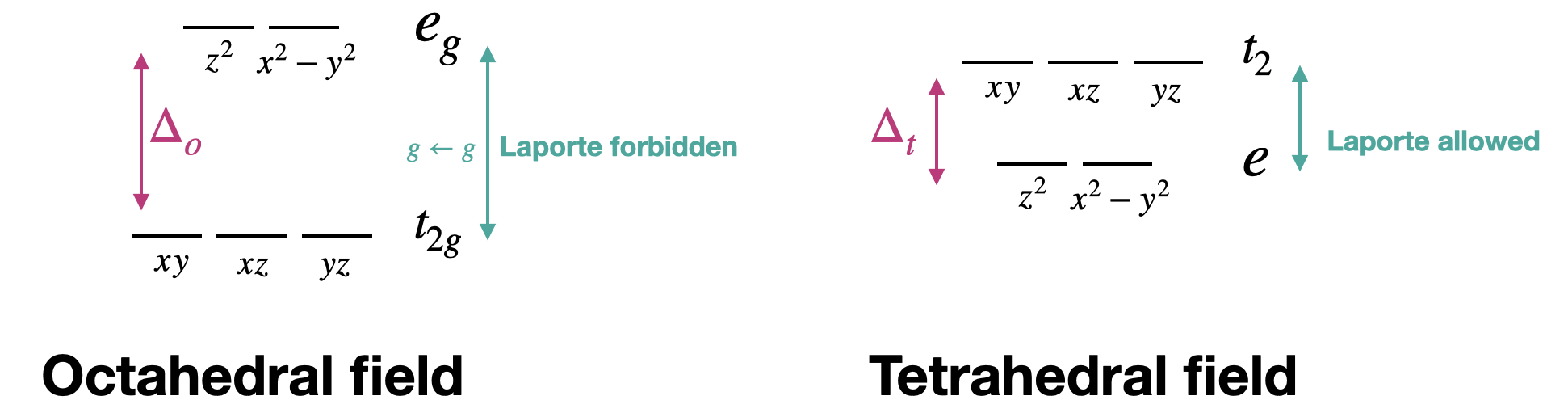
Tetrahedral metal complexes often have more intense electronic transitions than their octahedral counterparts. This is due to the fact that the \(d-d\) transitions in a tetrahedron are allowed by the Laporte selection rule, while \(d-d\) transitions in an octahedral complex are Laporte-forbidden. Recall that the Laporte selection rule applies to centrosymmetric complexes only. The Laporte rule applies to octahedral complexes but not to tetrahedral complexes because a tetrahedron does not have a center of inversion. Notice that the terms (and orbital labels) in a tetrahedron do not include the \(g\) subscripts that are present under octahedral symmetry (Figure \(\PageIndex{1}\)). The splitting pattern of a tetrahedral complex is exactly opposite to the octahedral case. In the case of a tetrahedron, however, the "\(g\)" subscripts are inappropriate because of the tetrahedron's lack of a center of inversion, and transitions between the terms in a tetrahedron do not violate the Laporte Rule.
Another way to explain this in terms of electron transitions between orbitals is through the orbital mixing required to form a tetrahedral complex. Orbital types (i.e., \(s,p,d\)) must mix to form the moleculare orbitals of a tetrahedral transition metal complex. The mixing of \(s\) and \(p\) orbitals with the \(d\) orbitals allows transitions that are forbidden in the case of pure d-orbitals.
It is also worth noting that \(\Delta_t = \frac{4}{9} \Delta_o\). The smaller \(\Delta\) for transition metals means that the tetrahedral complexes can absorb at a lower energy and longer wavelength relative to an analogous octahedron.
Tanabe-Sugano diagrams for tetrahedral complexes
Due to the opposite splitting pattern, the transitions for a \(d^n\) tetrahedral complex are sufficiently represented by the \(d^{10-n}\) Tanabe-Sugano diagram (just drop the \(g\) subscripts from the diagrams). For example, the electronic spectrum of a \(d^8\) tetrahedral complex (e.g., \(\ce{[Ni(H2O)6]^2+}\)) can be interpreted using the \(d^2\) Tanabe-Sugano diagram.


