11.1.1: Beer-Lambert Absorption Law
- Page ID
- 281609
When light passes through a solution that absorbs light, it enters the solution with an initial intensity (\(I_o\)) at a given wavelength, and it emerges with an intensity, \(I\).
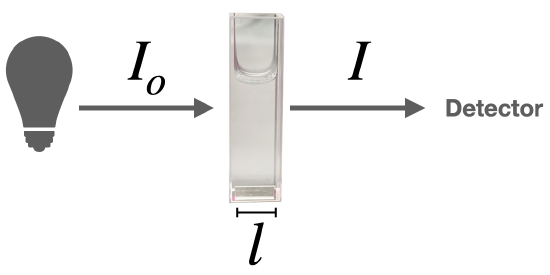
The Beer-Lambert Law defines the relationship between absorbance at a given wavelength and the concentration of the solution.
\[\log \left( \frac{I_{o}}{I} \right)=A=\varepsilon l c\]
The absorbance (A) is a unitless number because \(\frac{I_{o}}{I}\) is unitless. The absorbance depends on the concentration (\(c\)) and the path length (\(l\)). The concentration of the sample solution is measured in molarity (M) and the length of the light path in centimeters (cm). The Greek letter epsilon (\(\varepsilon\)) in these equations is called the molar absorptivity (also called the molar absorption coefficient). The units of \(\varepsilon\) are \(\frac{L}{mol \times cm}\) or \(L \times mol^{-1}\times cm^{-1}\).
Chemists most often measure and report absorbed light in terms of wavelength (\(\lambda\)) in units of nanometers (nm). But the wavelength scale is inconvenient for measuring energy because it is inversely proportional to both frequency and energy. In other disciplines, like physics for example, absorption spectra are more often reported in terms of frequency (\(\nu\)) using units of inverse centimeters (\(cm^{-1}\)). The relationships between energy (E), \(\nu\), and \(\lambda\) are given by the equation below:
\[E=h v=\frac{h c}{\lambda}=h c\left(\frac{1}{\lambda}\right)=h c \bar{v}\]


