8.6.2: Heavier Elements of Group 13 and the Inert Pair Effect
- Page ID
- 167520
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The group 13 metals also form conventional compounds as well as M-X-M type bridge bonds and metal-like clusters.
The group 13 metals form a wide variety of compounds that may be explained in terms of ionic or pairwise covalent bonding. These oxides can generally be explained as ionic. Thallium's main oxide, Tl2O, crystallizes in the CdI2 lattice, and consists of layers of edge-linked octahedra, as shown in Figure \(\sf{\PageIndex{1A}}\), while the most stable form of Al2O3 , corundum, consists of layers of edge-linked AlO6 octahedra vertex-linked to adjacent layers as shown in Figure \(\sf{\PageIndex{1B}}\).

Nevertheless, although it is possible to explain the structural chemistry of Al2O3 as involving an ionic lattice, Al does form covalently-bonded oxyanions just as boron does. A particularly well known example is the Al6O1818- anion, which contains AlO4 tetrahedra covalently vertex-linked into an Al6O6 ring as shown in Scheme \(\sf{\PageIndex{I}}\). The calcium salt of this anion is found in tricalcium aluminate cements, which as the name suggests are formed by mixing 3 equivalents of CaO with one of Al2O3.
Scheme \(\sf{\PageIndex{I}}\). Two views of the Al6O1818- anion.


The aluminum trihalides are effective Lewis acids. This property serves as the basis for their use as a catalyst in Friedel-Crafts alkylations and acylations. In these reactions, AlCl3 acts as a Lewis base to abstract a chloride ion from an alkyl or acyl halide, generating a carbon-based cation that can add to an aromatic ring in an electrophilic aromatic substitution reaction (Scheme \(\sf{\PageIndex{II}}\)).
Scheme \(\sf{\PageIndex{II}}\). AlCl3 acts as a Lewis acid catalyst in facilitating Friedel Crafts acylation.

Trialkyl group 13 compounds also exist as monomers and dimers, although the monomer-dimer preference increasingly shifts towards monomer on going from Al to In. In general, GaR3 and InR3 species exist as monomers, while for Al the monomer and dimer are often in equilibrium (Scheme \(\sf{\PageIndex{II}}\)) for smaller alkyl groups, shifting towards monomer with bulky ones.
Scheme \(\sf{\PageIndex{II}}\). Dimer-monomer equilibrium of trimethyl aluminum.

As with their boron analogues, trialkyl aluminum and trialkylgallium compounds are effective Lewis acids, reacting with Lewis bases at the group 13 element to give tetrahedral species.
The low electronegativity of the group 13 metals also means that the alkyl groups of group 13 trialkyl complexes are nucleophilic and basic. This reactivity is employed in the commercial use of GaR3 species for the chemical vapor deposition of semiconductor-grade GaAs via reaction of GaR3 with AsH3.
\[\sf{Ga(CH_3)_3~~+~~AsH_3~~\longrightarrow~~GaAs(s)~~+~~3~CH_4} \nonumber \]
Compounds containing Al-Al and Ga-Ga single and multiple bonds can be prepared, although Al≡Al bonds have so far only been demonstrated in the gas phase.1 In the case of Ga, multiple bonds can be formed by reduction of compounds of type RGaX2 by using extremely bulky R groups to prevent dimerization of any Ga=Ga or Ga≡Ga species formed. The nature of the multiply-bonded species formed this way have been the subject of much theoretical investigation. The 1997 report of the synthesis of a gallyne2 in particular set off a storm of controversy about the nature of its Ga-Ga bond, leading to competing claims that the bond should be understood as involving single, triple, or even double bond interactions. Some of the options are depicted in Figure \(\sf{\PageIndex{3A}}\). Although no clear consensus has yet emerged, recent experimental and theoretical work3 has pointed to the description of bonding as involving a \(\sigma\) bond, a conventional \(\pi\) bond, and a Klinkhammer-type slipped \(\pi\) bond, as shown in Figure \(\sf{\PageIndex{3}}\).

Aluminum and gallium are also known to form atomic clusters. There are two types:
- Naked metal clusters
Examples include anions like Tl77- , Ga117- , and Tl1311- formed by reaction of the metal or metal halide with a powerful reductant (usually an alkali or similar highly reducing metal) and "magic clusters" containing unusually stable species like Al7+ and Al13- formed by laser sputtering of aluminum (i.e., irradiating an Al surface with a laser and watching what sort of clusters come off). Among these, Al13- is so stable that it can even be prepared by solution phase reduction of dendrimer-encapsulated AlCl3 by ketyl radicals. The chemical behavior of the Al13- anion may be rationalized by thinking about it as analogous to a "halide," specifically of the superatom Al13. Superatoms like Al13 are clusters that possess well-defined energy levels and directional valence orbitals just as atoms do; consequently, they exhibit atom-like reactivity in tending to gain, lose, or share electrons to fill a shell of low energy orbitals. In the case of Al11, its reactivity mimics that of the halogens and so it is described as a superhalogen.
Examples of naked metal cluster structures are given in Figure \(\sf{\PageIndex{4}}\).

As may be seen from the structures in Figure \(\sf{\PageIndex{4}}\), these clusters adopt shapes similar to those of the boranes, although as is the case with Tl1311- and Al13- sometimes one of the metal atoms resides in the cluster interior. As might be expected, the stability of many of these clusters is analogous to that of the boranes in being explicable in terms of Wade's rules, although given that Tl1311- and Al13- are isostructural and both stable while differing in their valence electron counts by ten electrons it is clear that this is not always the case.
The existence of multiple stable electron counts in group 13 superatom clusters like Al13- has been explained by the jellium model of cluster stability, which treats clusters like Al13- as consisting of an "ionic core" consisting of the nuclei and their core electrons (Al3+)13 surrounded by the "jelly" of valence electrons. In the case of Al13 clusters, this model gives stable sets of energy levels allowing for configurations containing the "magic numbers" of 2, 8, 18, 20, 34, or 40 electrons. In this model, the 40 valence electrons in Al13- correspond to the last of these stable states. Thus Tl1311- and Al13- represent different regimes of stability, one consistent with a Wade-type cluster similar to the boranes explicable in terms of molecular orbitals formed by combinations of atomic orbitals and the other one explicable in terms of the orbitals given by treating Al13 as a superatom in the jellium model.
- Metalloid clusters
Metalloid clusters consist of a small particle of metal surrounded by ligands. Some such Al and Ga clusters even contain upwards of 69 atoms. Examples include clusters with formulas [Al69{N(SiMe3)2}18]3- and [Al77N(SiMe3)2}20]2-. The structure of a relatively small example is given in Figure \(\sf{\PageIndex{5}}\).

Although the group 13 metals have varying tendencies to form covalent bonds, they tend to act chemically as metals.
Some representative features of these elements illustrative of their chemistry are summarized in Table \(\sf{\PageIndex{1}}\).
Table \(\sf{\PageIndex{1}}\). Illustrative properties of the group 13 metals.9,10
| Group 13 Metal | Natural Source |
Oxides (major product of reaction of the element with O2 is given in bold) |
Illustrative Stable Mononuclear Halides | Redox Behavior in Acidic Solution |
| Aluminum, Al |
Aluminosilicates like
|
Al2O3 | AlX3 (X = F, Cl, Br, I) |  |
| Gallium, Ga |
As impurities in Aluminum and Fe/Zn ores
Small amounts in Gallite, CuGaS2 |
Ga2O3 Ga2O |
Network covalent GaF3 Dimeric Ga2X6 (X = Cl, Br, I) GaIGaIII2Cl7 GaIGaIIIX4 (X = Cl, Br, I) |
 |
| Indium, In |
As impurities in Zn and Cu/Fe ores
|
In2O3 |
InX3 (X = F, Cl, Br, I) InI3(InIIIX6) (X = Cl, Br) InIInIIIX4 (X = Br, I) InX (X = Cl, Br, I) |
 |
| Thallium, Tl |
As impurities in sulfide-rich ores
small amounts in minerals like TlCu7Se4, TlPbAs5S9, others |
Tl2O3 Tl2O |
TlF3 TlX (X = F, Cl, Br, I) thallium(I) triiodide, TlI(I3) |
 |
As may be seen from the data in Table \(\sf{\PageIndex{1}}\), on moving down group 13 from Al to Tl
- Geologic occurrence of the elements in oxide ores (Al, Ga) becomes increasingly replaced by a preference for occurrence in sulfides and selenides (Ga, In, Tl). This is consistent with increasing softness on moving down group 13.
- There is an increasing preference for the +1 oxidation state. This may be seen from
- the standard potentials for reduction of M3+ and M+ to the metal. These show a decrease in the stability of the +3 ion on going down the group. In the case of Tl, Tl3+(aq) is even less stable than Tl+(aq).
- an increase in prevalence of the +1 oxidation state in the monomeric halides. The aluminum monohalides, AlX, are highly unstable; the bromide and chloride of GaI stabilizable (but perhaps not monomeric); the InI halides stable only for the less oxidizing halogens Cl, Br, and I; and for thallium, TlF3 is the only stable Tl3+ halide.
This increasing preference for an oxidation state two lower than maximum valence is not restricted to the group 13 elements. It is a common feature of post transition element chemistry that these elements can act as if their ns2 valence electrons are inert, on account of which this tendency has been referred to somewhat misleadingly as the inert pair effect.
Post-transition metals exhibit the inert pair effect, in which they act as if their ns2 valence electrons do not contribute to bonding.
Metals and metalloids of the p block commonly possess two stable oxidation states, with one corresponding to the loss of all their ns and np valence electrons and one the loss of two fewer electrons. This is evident from the well-known ions and oxidation states observed for the group 13, 14, and 15 metals and metalloids shown in Scheme \(\sf{\PageIndex{III}}\).
Scheme \(\sf{\PageIndex{III}}\). Common oxidation states of group 13, 14, and 15 metals and metalloids.

Moreover, there is an increasing preference for the lower oxidation state on going down a group in the periodic table so that
- The higher (n+) oxidation state is favored for lighter elements so that the order of preference is
Al3+ > Ga3+ > In3+ > Tl3+
- The lower (n - 2)+ oxidation state is favored for heavier elements
Al+ < Ga+ < In+ < Tl+
This is why the most stable oxide and chloride of Al are Al2O3 and AlCl3 while the most stable oxides and Cl of Tl are Tl2O and TlCl.
The classical explanation for the existence of this behavior is to postulate that the heavier elements' ns2 valence electrons are chemically inert - i.e., an inert pair. For this reason the observation that many post transition elements have stable n+ and (n-2)+ oxidation states and that there is an increasing preference for the lower oxidation state on moving down a group has been termed the inert pair effect.
The inert pair effect is due to the decrease in bond energies down a group.
However, the term inert pair effect is a misnomer. There are two reasons for this
- The ns electrons do not become significantly more inert (a.k.a. lower in energy) as one descends the groups of the periodic table. A cursory look at the valence s and p orbital energies of the main group elements reveals that it is simply not the case that the ns orbitals become lower in energy on going down a group of the periodic table. As may be seen from the energies given in Figure \(\sf{\PageIndex{6}}\), the energy of the ns orbitals is generally lowest for period 1 and 2 elements, after which the only overall trend that may be noted is that the ns orbital energies of the rows 3-6 elements generally do not differ by more than 20% across the entire range.

2. The inert pair effect is due to the decrease in bond energies as bond lengths increase down a group of the periodic table. To see how this leads to the appearance of ns electron inertness as one descends the periodic table, consider that the process of forming an E-X bond generally involves
- endergonic oxidation of the metal
- exergonic formation of a M-X bond
Consider the interplay between these two energies in the case of forming the monohalides and trihalides of the group 13 elements. The cost of oxidation of the metal reflects a balance between the ionization energies given in Table \(\sf{\PageIndex{2}}\) and bond energies given in Table \(\sf{\PageIndex{3}}\).15
Table \(\sf{\PageIndex{2}}\). Ionization energy costs for formation of M+ and M3+. Recalculated from the similar table of ionization energies at en.Wikipedia.org/wiki/Inert_pair_effect.
| Process | B | Al | Ga | In | Tl |
|
M \(\rightarrow\) M+ IE1 (kJ/mol) |
800 | 577 | 578 | 558 | 589 |
|
M \(\rightarrow\) M3+ IE1 + IE2 + IE3 (kJ/mol) |
6886 | 5137 | 5520 | 5082 | 5438 |
|
Inert pair oxidation: M+ \(\rightarrow\) M3+ IE2 + IE3 (kJ/mol) |
6,086 | 4,560 | 4,942 | 4,524 | 4,849 |
Table \(\sf{\PageIndex{3}}\). M-Cl homolytic bond energies in kJ/mol.15
| Bond | B-Cl | Al-Cl | Ga-Cl | In-Cl | Tl-Cl |
| Typical E-Cl Bond Dissociation energy (kJ/mol) | 536 | 494 | 481 | 439 | 372.8 |
As may be seen from Table \(\sf{\PageIndex{3}}\), the energy cost for ionization of the "inert pair" is greatest for boron, drops by ~25% on going to Al, and then remains approximately constant for the remaining members of the group. In contrast, the compensating bond dissociation energies drop more slowly on going from B to Tl, with successive ~10% and ~20% decreases on going from Ga to In and In to Tl. Because bond energies drop on going from Al to Tl while ionization energies do not, it is thought that the inert pair effect isn't due to the inertness of the ns electrons but rather due to the weakening of bond energies. When the stabilization energy due to M-E bond formation is no longer enough to pay the cost needed to oxidize away the ns electrons, the lower oxidation state will be more stable than the higher one, just as it would if the ns electrons really were inert.
The inert pair of electrons exerts structural effects.
Regardless of the origin of the inert pair effect, the inert pair of electrons influences the structure of compounds in the (n-2)+ oxidation state. Electron pairs that do this by altering the observed geometry of compounds are said to be stereochemically active inert pairs. The lone pairs of SnIICl2 are the classic example. As shown in Figure \(\sf{\PageIndex{7A}}\), gas phase SnIICl2 exhibits an AX2E VSEPR geometry, while SnIICl2 in its solid state, hydrates, and salts all exhibit AX3E geometries, consistent with the influence of a lone pair of electrons.

Sometimes the coordination geometry of an element is exactly that which would be expected if the inert pair were not present. In such cases the inert pair is said to be stereochemically inert. In general, when the element's coordination number is less than six, the inert pairs will be stereochemically active, while for coordination numbers of six or more the pair may be stereochemically active or stereochemically inert.
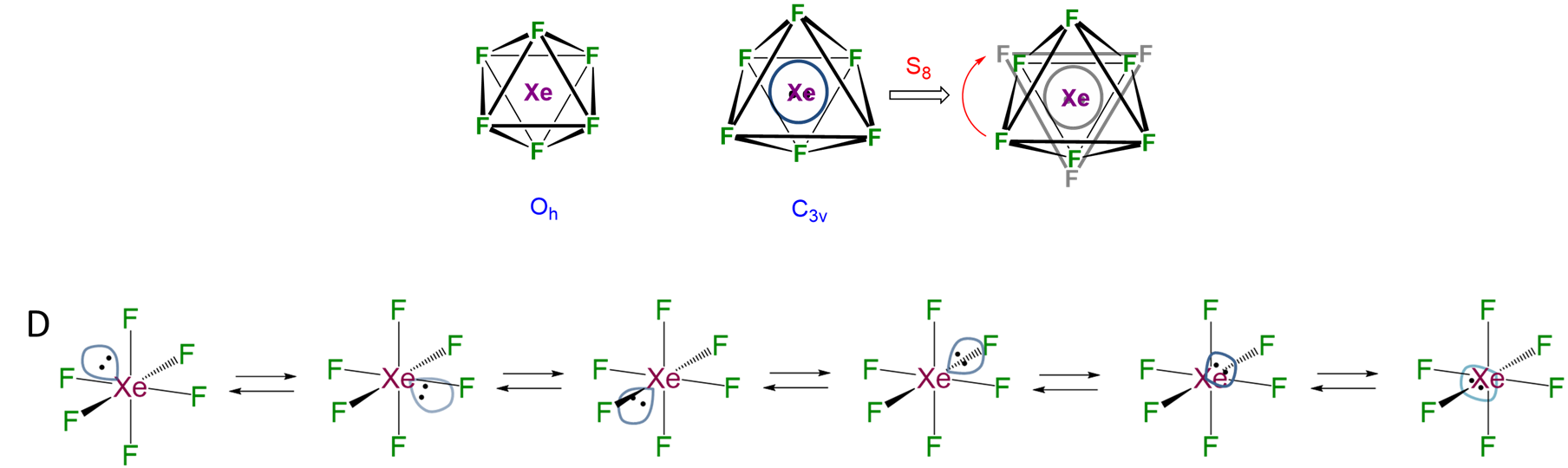
Even when an inert pair appears stereochemically inert, it does not mean that it exerts no effect on the structure of the compound. It may be that the pair is simply fluxional and only appears to be inert because it is rapidly adjusting its position among possible locations. For instance, in the structure of BiI3 shown in Figure \(\sf{\PageIndex{7B}}\), the lone pair is likely interchanging between the six faces of the octahedron defining the coordination sphere, as is the case for XeF6 (Figure \(\sf{\PageIndex{8}}\)).
Notes and References
1. Zhang, X.; Popov, I. A.; Lundell, K. A.; Wang, H.; Mu, C.; Wang, W.; Schnöckel, H.; Boldyrev, A. I.; Bowen, K. H., Realization of an Al≡Al Triple Bond in the Gas-Phase Na3Al2− Cluster via Double Electronic Transmutation. Angewandte Chemie International Edition 2018, 57 (43), 14060-14064.
2. Su, J.; Li, X.-W.; Crittendon, R. C.; Robinson, G. H., How Short is a -Ga⋮Ga- Triple Bond? Synthesis and Molecular Structure of Na2[Mes*2C6H3-Ga⋮Ga-C6H3Mes*2] (Mes* = 2,4,6-i-Pr3C6H2): The First Gallyne. Journal of the American Chemical Society 1997, 119 (23), 5471-5472.
3. Kobera, L.; Southern, S. A.; Rao, G. K.; Richeson, D. S.; Bryce, D. L., New Experimental Insight into the Nature of Metal−Metal Bonds in Digallium Compounds: J Coupling between Quadrupolar Nuclei. Chemistry – A European Journal 2016, 22 (28), 9565-9573.
4. Klinkhammer, K. W., How Can One Recognize a Triple Bond between Main Group Elements? Angewandte Chemie International Edition in English 1997, 36 (21), 2320-2322.
5. Kambe, T.; Haruta, N.; Imaoka, T.; Yamamoto, K., Solution-phase synthesis of Al13− using a dendrimer template. Nature Communications 2017, 8 (1), 2046.
6. Schnepf A. (2016) Metalloid Clusters. In: Dehnen S. (eds) Clusters – Contemporary Insight in Structure and Bonding. Structure and Bonding, vol 174. Springer, 135-200.
7. Schnöckel, H., Structures and Properties of Metalloid Al and Ga Clusters Open Our Eyes to the Diversity and Complexity of Fundamental Chemical and Physical Processes during Formation and Dissolution of Metals. Chemical Reviews 2010, 110 (7), 4125-4163.
8. Köhnlein, H.; Purath, A.; Klemp, C.; Baum, E.; Krossing, I.; Stösser, G.; Schnöckel, H., Synthesis and Characterization of an Al693- Cluster with 51 Naked Al Atoms: Analogies and Differences to the Previously Characterized Al772- Cluster. Inorganic Chemistry 2001, 40 (19), 4830-4838.
9. The list of halides are selected from those listed in references 4-6 and element halide Wikipedia pages, checked against the original literature. The specific pages used include en.Wikipedia.org/wiki/Aluminium_halide, en.Wikipedia.org/wiki/Gallium_halides, en.Wikipedia.org/wiki/Indium_halides, and en.Wikipedia.org/wiki/Thallium_halides.
10. All potentials are taken from Bard, A. J.; Parsons, R.; Jordan, J. Standard potentials in aqueous solution. M. Dekker: New York, 1985. Note that this reference reports a Ga2+/0 potential in addition to the Ga3+/0 potential given. This couple was not included since the nature of the Ga2+ species referenced (GaCl2 produced by reacting Ga and GaCl3) was unclear (i.e., it may be a GaIGaIII species or possess a Ga-Ga bond).
11. Greenwood, N. N.; Earnshaw, A., Chemistry of the elements. 2nd ed.; Butterworth-Heinemann: Oxford ; Boston, 1997.
12. Cotton, F. A.; Cotton, F. A., Advanced inorganic chemistry. 6th ed.; Wiley: New York, 1999; p xv, 1355 p.
13. Pardoe, J.; Downs, A., Development of the Chemistry of Indium in Formal Oxidation States Lower than +3 †. Chemical reviews 2007, 107, 2-45.
14. Aldridge, S.; Downs, A. J. The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, Wiley, 2011.
15. Bond energies are taken from Table 4.11 of Dean, J. A.; Lange, N. A., Lange's handbook of chemistry, 14th ed. McGraw-Hill: New York, 1992.
Contributors and Attributions
Stephen Contakes, Westmont College

