11.1: The Group 18 Elements- The Noble Gases
- Page ID
- 212679
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The elements
The Group 18 elements have a particular name Noble gases. Noble gas is translated from the German noun Edelgas, first used in 1898 by Hugo Erdmann (1862 - 1910) to indicate their extremely low level of reactivity. The noble gases were often also called the inert gases, however, since noble gas compounds are now known this name is no longer used. Table \(\PageIndex{1}\) lists the derivation of the names of the Noble gases.
| Element | Symbol | Name |
| Helium | He | Greek helios meaning the Sun |
| Neon | Ne | From the Greek meaning new one |
| Argon | Ar | From the Greek meaning inactive |
| Krypton | Kr | From the Greek kryptos meaning the hidden one |
| Xenon | Xe | From the Greek xenos], meaning foreigner, stranger, or guest |
| Radon | Rn | From its radioactive nature |
Discovery
Helium
The first evidence of helium was the observation by astronomer Pierre Janssen (Figure \(\PageIndex{1}\)) on August 18, 1868 as a bright yellow line with a wavelength of 587.49 nm in the spectrum of the chromosphere of the Sun. On October 20 of the same year, English astronomer Norman Lockyer (Figure \(\PageIndex{2}\)) observed a yellow line in the solar spectrum, which he named the D3 Fraunhofer line because it was near the known D1 and D2 lines of sodium. He concluded that it was caused by an element in the Sun unknown on Earth. Lockyer and Edward Frankland (Figure \(\PageIndex{3}\)) named the element with the Greek word for the Sun, helios.



On March 26, 1895 British chemist Sir William Ramsay (Figure \(\PageIndex{4}\)) isolated helium on Earth by treating the mineral cleveite (a radioactive mineral containing uranium and found in Norway) with mineral acids.

Neon
Neon was discovered in 1898 by Sir William Ramsay (Figure \(\PageIndex{4}\)) and Morris Travers (Figure \(\PageIndex{5}\)). When Ramsay chilled a sample of air until it became a liquid, then warmed the liquid and captured the gases as they boiled off. After nitrogen, oxygen, and argon, the three gases that boiled off were krypton, xenon, and neon.

Argon
In 1785 Henry Cavendish (Figure \(\PageIndex{6}\)) suspected that argon was present in air but it was not isolated until 1894 by Lord Rayleigh (Figure \(\PageIndex{7}\)) and Sir William Ramsay (Figure \(\PageIndex{4}\)) in an experiment in which they removed all of the oxygen, carbon dioxide, water and nitrogen from a sample of clean air.

Krypton
Krypton was discovered in 1898 by Sir William Ramsay (Figure \(\PageIndex{4}\)) and Morris Travers (Figure \(\PageIndex{5}\)) in residue left from evaporating nearly all components of liquid air.
Note
In 1960, an international agreement defined the meter (m) in terms of wavelength of light emitted by the 86Kr isotope (wavelength of 605.78 nm). This agreement replaced the standard meter located in Paris, which was a metal bar made of a Pt-Ir alloy, and was itself replaced by a definition based on the speed of light, a fundamental physical constant. In October 1983, the Bureau International des Poids et Mesures defined the meter as the distance that light travels in a vacuum during 1/299,792,458 s.
Xenon
Xenon was discovered by William Ramsay (Figure \(\PageIndex{4}\)) and Morris Travers (Figure \(\PageIndex{5}\)) on July 12, 1898, shortly after their discovery of krypton and neon.
Radon
Radon was the fifth radioactive element to be discovered after uranium, thorium, radium and polonium. Discovered in 1900 by Friedrich Dorn (Figure \(\PageIndex{8}\)) after he noticed that radium compounds emanate a radioactive gas that he named Radium Emanation (Ra Em). Prior to these experiments, in 1899, Pierre and Marie Curie (Figure \(\PageIndex{9}\)) observed that the gas emitted by radium remained radioactive for a month. Later that year, Ernest Rutherford (Figure \(\PageIndex{10}\)) noticed variations when trying to measure radiation from thorium oxide. In 1901, he demonstrated that the emanations are radioactive, but credited the Curies for the discovery of the element.


Abundance
The abundance of the Noble gases is given in Table \(\PageIndex{2}\).
| Element | Terrestrial abundance (ppm) |
| He | 8 x 10-3 (Earth’s crust), 4 x 106 (sea water), 5 (atmosphere) |
| Ne | 70 x 10-3 (Earth’s crust), 0.2 (sea water), 18 (atmosphere) |
| Ar | 1.2 (Earth’s crust), 0.45 (sea water), 0.93 x 104 (atmosphere) |
| Kr | 10 x 10-6 (Earth’s crust), 80 x 10-6 (sea water), 1 (atmosphere) |
| Xe | 2 x 10-6 (Earth’s crust), 100 x 10-6 (sea water), 90 x 10-3 (atmosphere) |
Isotopes
The naturally abundant isotopes of the Group 18 elements are listed in Table \(\PageIndex{3}\). All of the isotopes of radon are radioactive.
| Isotope | Natural abundance (%) |
| Helium-3 | 0.000137 |
| Helium-4 | 99.999863 |
| Neon-20 | 90.48 |
| Neon-21 | 0.27 |
| Neon-22 | 9.25 |
| Argon-36 | 0.337 |
| Argon-86 | 0.063 |
| Argon-40 | 99.600 |
| Krypton-78 | 0.35 |
| Krypton-80 | 2.25 |
| Krypton-81 | trace |
| Krypton-82 | 11.6 |
| Krypton-83 | 11.5 |
| Krypton-84 | 57 |
| Krypton-86 | 17.3 |
| Xenon-124 | 0.095 |
| Xenon-126 | 0.089 |
| Xenon-128 | 1.91 |
| Xenon-129 | 26.4 |
| Xenon-130 | 4.07 |
| Xenon-131 | 21.2 |
| Xenon-132 | 26.9 |
| Xenon-134 | 10.4 |
| Xenon-136 | 8.86 |
| Radon-222 | trace |
Unlike most elements, helium's isotopic abundance varies greatly by origin, due to the different formation processes. The most common isotope, 4He, is produced on Earth by a decay of heavier radioactive elements. It was also formed in enormous quantities during the Big Bang.
Naturally occurring 40K with a half-life of 1.25 × 109 years, decays to stable 40Ar (11.2%) by electron capture and positron emission, and also to stable 40Ca (88.8%) via beta decay. These properties and ratios are used to determine the age of rocks.
With a half-life of 230,000 years 81Kr is used for dating 50,000 - 800,000 year old groundwater. 85Kr is an inert radioactive noble gas with a half-life of 10.76 years. It is produced in nuclear bomb testing and nuclear reactors. 85Kr is released during the reprocessing of fuel rods from nuclear reactors.
Industrial production of the elements
Helium is extracted by fractional distillation from natural gas, which contains up to 7% helium. Since helium has a lower boiling point than any other element, low temperature and high pressure are used to liquefy nearly all the other gases. The resulting helium gas is purified by successive exposures to lowering temperatures. A final purification step with activated charcoal results in 99.995% pure Grade-A helium.
Argon is produced industrially by the fractional distillation of liquid air, a process that separates liquid nitrogen, which boils at 77.3 K, from argon, which boils at 87.3 K and oxygen, which boils at 90.2 K. Xenon is obtained commercially as a byproduct of the separation of air into oxygen and nitrogen.
Physical properties
The physical properties of the Group 18 elements are given in Table \(\PageIndex{4}\).
| Element | Mp (°C) | Bp (°C) |
| He | -272.20 | -268.93 |
| Ne | -248.59 | -246.08 |
| Ar | -189.35 | -185.85 |
| Kr | -157.36 | -153.22 |
| Xe | -111.7 | -108.12 |
| Rn | -71.15 | -61.85 |
All of the Noble gases show characteristic spectral lines (Figure \(\PageIndex{11}\) – Figure \(\PageIndex{15}\)).





Compounds of the Group 18 elements.
Only a few hundred noble gas compounds have been formed. Neutral compounds of helium and neon have not been formed, while xenon, krypton, and argon have shown only minor reactivity. The reactivity follows the order:

Xenon compounds are the most numerous of the noble gas compounds. Oxidation states of +2, +4, +6, and +8 with electronegative elements, e.g., XeF2, XeF4, XeF6, XeO4, and Na4XeO6. Compounds of xenon bound to boron, hydrogen, bromine, iodine, beryllium, sulphur, titanium, copper, and silver have also been observed but only at low temperatures in noble gas matrices, or in supersonic noble gas jets.
Although radon is more reactive than xenon it should form chemical bonds more easily than xenon, however, due to the high radioactivity and short half-life of radon isotopes, only a few fluorides and oxides of radon have been formed.
Krypton is less reactive than xenon, and oxidation states are generally limited to +2, KrF2. Compounds in which krypton forms a bond to nitrogen and oxygen are only stable below -60 °C and -90 °C, respectively. Krypton atoms chemically bound to other nonmetals (hydrogen, chlorine, carbon) as well as some late transition metals (copper, silver, gold), but only at low temperatures in noble gas matrices, or in supersonic noble gas jets. Similar conditions were used to obtain the first compounds of argon.
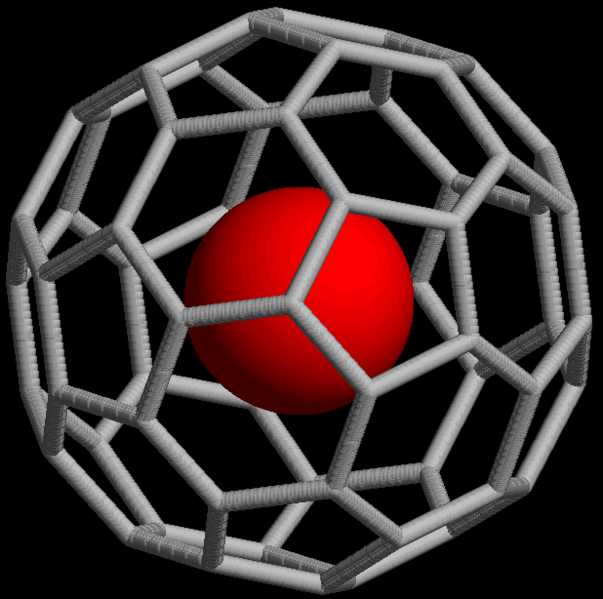
Noble gases also form non-covalent compounds, for example clathrates that consist of an atom trapped within cavities of crystal lattices of organic and inorganic compounds. Noble gases can form endohedral fullerene compounds, in which the noble gas atom is trapped inside a fullerene molecule (Figure \(\PageIndex{16}\)).
Bibliography
- L. Pauling, J. Am. Chem. Soc., 1933, 55, 1895.
- M. Saunders, H. A. Jiménez-Vázquez, R. J. Cross, and R. J. Poreda, Science, 1993, 259, 1428.


