8.1: The Group 15 Elements- The Pnictogens
- Page ID
- 212658
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The elements
The Group 15 elements have a particular name pnictogens. Despite the modern IUPAC notation, the Group 15 elements are still referred to as Group V elements in particular by the semiconductor industry. Table \(\PageIndex{1}\) lists the derivation of the names of the Group 15 elements.
| Element | Symbol | Name |
| Nitrogen | N | Latin nitrogenium, where nitrum (derived from Greek nitron) means saltpetre |
| Phosphorus | P | From the Greek phosphoros meaning bringer of light |
| Arsenic | As | Derived from Syriac zarniqa and Persian zarnikh, meaning yellow orpiment |
| Antimony | Sb | Greek anti and monos meaning not alone. The symbol Sb from Latin stibium |
| Bismuth | Bi | New Latin bisemutum from German Wismuth, meaning white mass |
Note
According to the Oxford English Dictionary, the correct spelling of the element is phosphorus. The word phosphorous is the adjectival form of the P3+ valence. In the same way that sulfur forms sulfurous and sulfuric compounds, phosphorus forms phosphorous compounds (e.g., phosphorous acid) and P5+ valency phosphoric compounds (e.g., phosphoric acids and phosphates).
Discovery
Nitrogen
Nitrogen was discovered by Rutherford (Figure \(\PageIndex{1}\)) in 1772. He called it noxious air or fixed air because there it had been known since the late 18th century that there was a fraction of air that did not support combustion. Nitrogen was also studied by Scheele (Figure \(\PageIndex{2}\)), Cavendish (Figure \(\PageIndex{3}\)), and Priestley (Figure \(\PageIndex{4}\)), who referred to it as burnt air or phlogisticated air.




Phosphorus
German alchemist Hennig Brand (Figure \(\PageIndex{5}\)) was experimenting with urine (which contains dissolved phosphates) in 1669. While attempting to create the fabled philosopher's stone (the legendary alchemical substance capable of turning base metals, such as lead, into gold) by the distillation of salts from urine, he produced a white material that glowed in the dark and burned with a brilliant light. He gave the substance the name phosphorus mirabilis (miraculous bearer of light). His process involved letting the urine stand for days then boiling it down to a paste which led to a white waxy substance, white phosphorus.

Brand sold the recipe for 200 thaler (a silver coin from whose name the dollar is derived) to D Krafft who toured much of Europe showing it. During his journeys he met Robert Boyle (Figure \(\PageIndex{6}\)) who without learning the details of the synthesis recreated and improved it by using sand in the reduction of the phosphate, (8.1.1).
\[\text{4 NaPO}_3 \text{ + 2 SiO}_2\text{ + 10 C} \rightarrow \text{2 Na}_2\text{SiO}_3\text{ + 10 CO + P}_4\]

Arsenic
Arsenic sulfides and oxides were known since ancient times. Zosimos (ca. 300 AD) describes roasting sandarach (realgar, α-As4S4) to obtain cloud of arsenious oxide (As2O3) that he reduced to metallic arsenic (Figure \(\PageIndex{7}\)).
Antimony
Antimony(III) sulfide, Sb2S3 was known as early as 3000 BC. Pastes of Sb2S3 powder in fat were used as eye cosmetics in the Middle East. An artifact made of antimony dating to about 3000 BC was found at Tello (part of present-day Iraq), and copper objects plated with antimony from 2500 - 2200 BC have been found in Egypt. The first European description of a procedure for isolating antimony is in the book De la pirotechnia by Vannoccio Biringuccio (1480 - 1539).
Bismuth
Since bismuth was known in ancient times, no one person is credited with its discovery. However, the French chemist Claude François Geoffroy (1729 - 1753) demonstrated in 1753 that this metal is distinct from lead and tin.
Abundance
The abundance of the Group 15 elements is given in Table \(\PageIndex{2}\).
| Element | Terrestrial abundance (ppm) |
| N | 25 (Earth’s crust), 5 (soil), 0.5 (sea water), 78 x 104 (atmosphere) |
| P | 1000 (Earth’s crust), 0.65 (soil), 60 x 10-3 (sea water), trace (atmosphere) |
| As | 1.5 (Earth’s crust), 10 (soil), 16 x 10-3 (sea water), trace (atmosphere) |
| Sb | 0.2 (Earth’s crust), 1 (soil), 0.3 x 10-3 (sea water) |
| Bi | 48 x 10-3 (Earth’s crust), 0.25 (soil), 400 x 10-6 (sea water) |
Isotopes
The naturally abundant isotopes of the Group 15 elements are listed in Table \(\PageIndex{3}\).
| Isotope | Natural abundance (%) |
| Nitrogen-14 | 99.634 |
| Nitrogen-15 | 0.0366 |
| Phosphorus-31 | 100 |
| Arsenic-75 | 100 |
| Antimony-121 | 57.36 |
| Antimony-123 | 42.64 |
| Bismuth-209 | 100% |
Two radioactive isotopes of phosphorus (32P and 33P) have half-lives that make them useful for scientific experiments (14.262 and 25.34 days, respectively). 32P is a β-emitter (1.71 MeV) and is used to produce radiolabeled DNA and RNA probes. Due to the high energy of the β particles which can penetrate skin and corneas, and because any 32P ingested, inhaled, or absorbed is incorporated into bone and nucleic acids extreme care needs to be taken in handling. The lower energy β particles emitted from 33P (0.25 MeV) make it useful for applications such as DNA sequencing.
While bismuth is traditionally regarded as the element with the heaviest stable isotope, 209Bi, it had long been suspected to be unstable on theoretical grounds. In 2003 researchers at the Institut d'Astrophysique Spatiale in Orsay, France, measured the alpha emission half-life of 209Bi to be 1.9 × 1019 years, over a billion times longer than the current estimated age of the universe!
Industrial production of the elements
Nitrogen is the largest constituent of the Earth's atmosphere (78.082% by volume, 75.3% by weight). It is created by fusion processes in stars, and is estimated to be the 7th most abundant element by mass in the universe. Industrial gas produced is by the fractional distillation of liquid air, or by mechanical means using gaseous air (i.e., pressurized reverse osmosis membrane or pressure swing adsorption). Commercial nitrogen is often a byproduct of air processing for industrial concentration of oxygen for steelmaking, etc.
White phosphorus was originally made commercially for the match industry in the 19th century, by distilling off phosphorus vapor from precipitated phosphates, mixed with ground coal or charcoal, (8.1.2). The precipitated phosphates were made from ground-up bones that had been de-greased and treated with strong acids. This process is, however, obsolete due to the submerged-arc furnace for phosphorus production was introduced to reduce phosphate rock. Calcium phosphate (phosphate rock) is heated to 1200 - 1500 °C with SiO2 and coke (impure carbon) to produce vaporized tetraphosphorus, P4.
\[\text{Ca}_{10}\text{(PO}_4\text{)}_6\text{F}_2\text{ + 15 C + 9 SiO}_2\rightarrow \text{6 P}_{\text{n(g)}}\text{ + 9 [(CaO.SiO}_2\text{)] + CaF}_2\text{ + 15 CO}_{\text{(g)}}\]
Physical properties
The physical properties of the Group 15 elements (Table \(\PageIndex{3}\)) encompasses a gas (N2), a non-metallic solid (P4), metalloids (As and Sb), and a metal (Bi).
| Element | Mp (°C) | Bp (°C) | Density (g/cm3) |
| N | -210.00 | -195.79 | 1.251 g/L (0 °C @ 101.325 kPa) |
| P | 44.2 (white), 610 (black) | 280.5 (white), 416 - 590 (sub., red), 620 (sub, violet) | 1.823 (white), 2.2 - 2.34 (red), 2.36 (violet), 2.69 (black) |
| As | 817 | 615 (sub.) | 5.727 |
| Sb | 630.63 | 1587 | 6.697 (solid), 6.53 (liquid) |
| Bi | 271.5 | 1564 | 9.78 (solid), 10.05 (liquid) |
Vapor phase
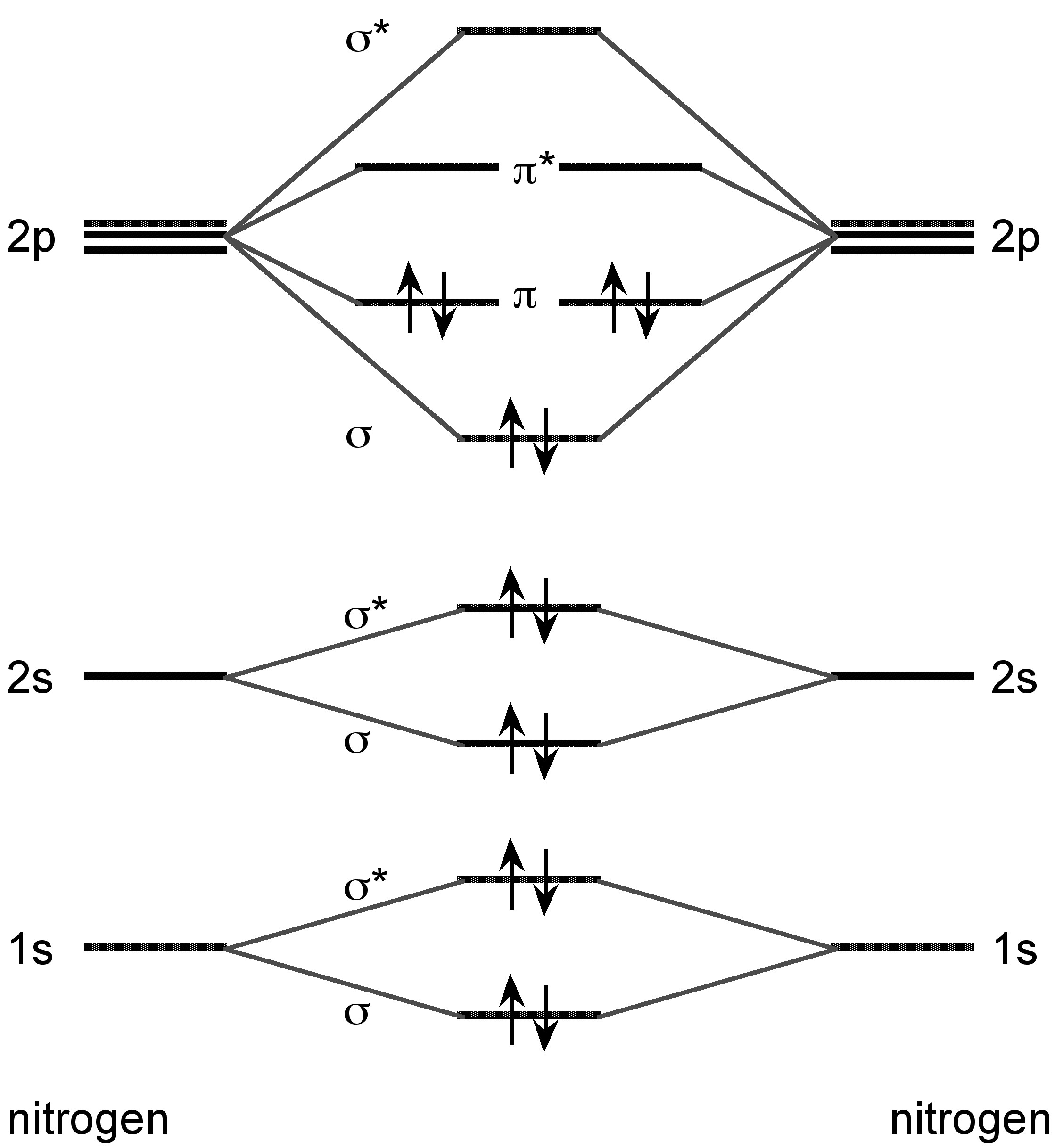
Nitrogen forms a dimer in the vapor phase with a triple bond (Figure \(\PageIndex{8}\)). In the vapor phase above 800 °C tetraphosphorus (P4) is partially dissociated to P2.
Solid state
Phosphorus forms a number of allotropes with very different properties (Figure \(\PageIndex{9}\)). Red phosphorus is an intermediate phase between the white and violet forms. Scarlet phosphorus is obtained by allowing a solution of white phosphorus in carbon disulfide to evaporate in sunlight. Black phosphorus is formed by heating white phosphorus under high pressures (ca. 12,000 atmospheres).

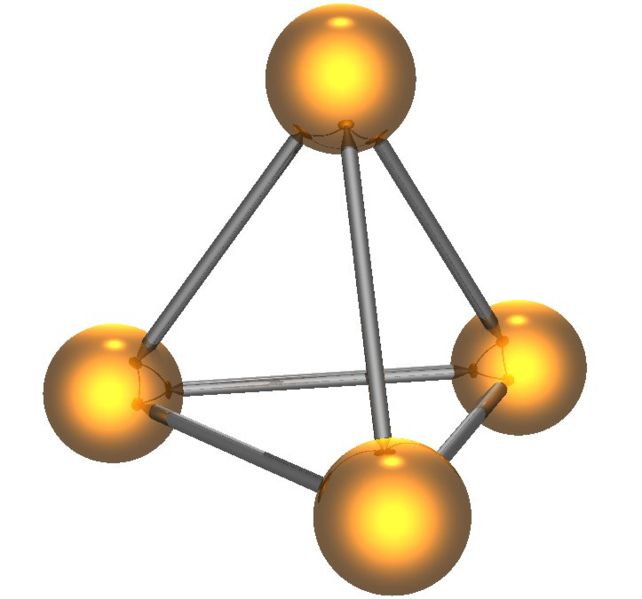
White phosphorus has two forms, low-temperature β form and high-temperature α form; both of which contain the P4 tetrahedron (Figure \(\PageIndex{10}\)). White phosphorus is the least stable, the most reactive, most volatile, less dense, and most toxic of the allotropes.
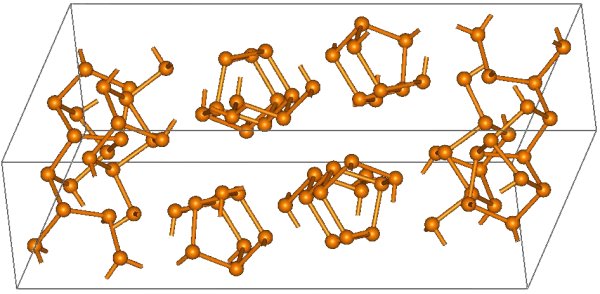
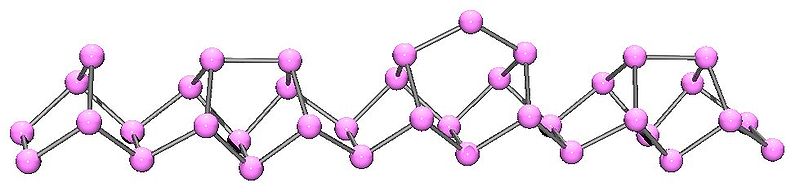
The structural relationship between white and red phosphorus involves breaking one of the P-P bonds in the P4 unit and forming a bond with a neighboring tetrahedron to give a chain structure (Figure \(\PageIndex{11}\)). Red phosphorus is formed by heating white phosphorus to 250 °C or by exposing white phosphorus to sunlight. Actually red phosphorus is not a single allotrope, but rather an intermediate phase between the white and violet phosphorus, and most of its properties have a range of values (Table \(\PageIndex{3}\)).
Violet phosphorus (Figure \(\PageIndex{12}\)) is the thermodynamic stable form of phosphorus that is produced by heating red phosphorus above 550 °C. Due to the synthesis being developed by Johann Hittorf (Figure \(\PageIndex{13}\)) it is sometimes known as Hittorf's phosphorus.

Black phosphorus is the least reactive allotrope and the thermodynamic stable form below 550 °C. It is also known as β-metallic phosphorus and has a structure somewhat resembling that of graphite (Figure \(\PageIndex{14}\)).
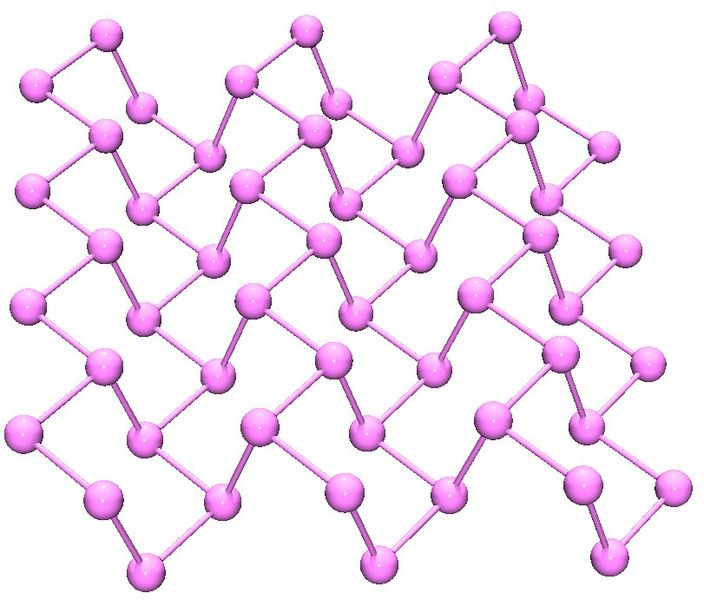
In a similar manner to phosphorus, arsenic has several allotropes some of which a structurally related to those of phosphorus. Grey arsenic has a structure similar to black phosphorus (Figure \(\PageIndex{10}\)). Yellow arsenic (As4) is soft and waxy with a structure similar to too P4 (Figure \(\PageIndex{14}\)). Finally, black arsenic is similar in structure to red phosphorus (Figure \(\PageIndex{11}\)). Antimony and bismuth are both traditional metals and have trigonal hexagonal structures (a = 4.299, c = 11.25 Å, and a = 4.537, c = 11.838 Å, respectively).
Bibliography
- V. Biringuccio, The Pirotechnia of Vannoccio Biringuccio: The Classic Sixteenth-Century Treatise on Metals and Metallurgy, Dover Publications (1990).


