6.14: Group 13 Halides
- Page ID
- 212890
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Trihalides, MX3
As shown in Table \(\PageIndex{1}\) all the combinations of Group 13 element (M) and halogen (X) exist for the trihalides (MX3), except thallium(III) iodide. It should be noted that while there is a compound with the general formula TlI3, it is actually a thallium(I) compound of I3-.
| Element | Mp (°C) | Bp (°C) |
| BF3 | -126.8 | -100.3 |
| BCl3 | -107.3 | 12.6 |
| BBr3 | -46.3 | 91.3 |
| BI3 | 49.9 | 210 |
| AlF3 | 1291 | - |
| AlCl3 | 192.4 (anhydrous), 0.0 (hexahydrate) | 120 (hexahydrate) |
| AlBr3 | 97.8 | 265 |
| AlI3 | 189.4 (anhydrous) 185 dec. (hexahydrate) | 300 subl. |
| GaF3 | 800 | 1000 |
| GaCl3 | 77.9 | 201 |
| GaBr3 | 121.5 | 278.8 |
| GaI3 | 212 | 345 |
| InF3 | 1172 | - |
| InCl3 | 586 | 800 |
| InBr3 | 220 | - |
| InI3 | 210 subl. | - |
| TlF3 | 300 dec. | - |
| TlCl3 | 40 dec. | - |
| TlBr3 | 40 dec. | - |
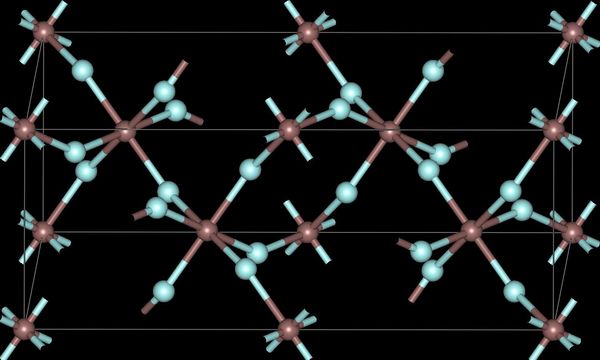
The trihalides of boron are all monomers with a coordination number of 3 (Table \(\PageIndex{2}\)), as evidence from their low melting points (Table \(\PageIndex{1}\)). In contrast, the fluorides and chlorides of the heavier Group 13 elements (except GaCl3) are generally ionic or have a high ionic character, with a coordination number of 6 (Table \(\PageIndex{2}\), Figure \(\PageIndex{1}\) and Figure \(\PageIndex{2}\)). The bromides and iodides (except InBr3) are generally dimeric with a coordination number of 4 (Table \(\PageIndex{2}\)) and have molecular structures involving halide bridging ligands (Figure \(\PageIndex{3}\) and Table \(\PageIndex{3}\)). AlCl3 is unusual in that in the solid state it has an ionic structure, but it is readily sublimed, and in the vapor phase (and liquid phase) it has a dimeric structure (Figure \(\PageIndex{3}\)).
| Element | Fluoride | Chloride | Bromide | Iodide |
| B | 3 | 3 | 3 | 3 |
| Al | 6 | 6 (4) | 4 | 4 |
| Ga | 6 | 4 | 4 | 4 |
| In | 6 | 6 | 6 | 4 |
| Tl | 6 | 6 | 4 | - |
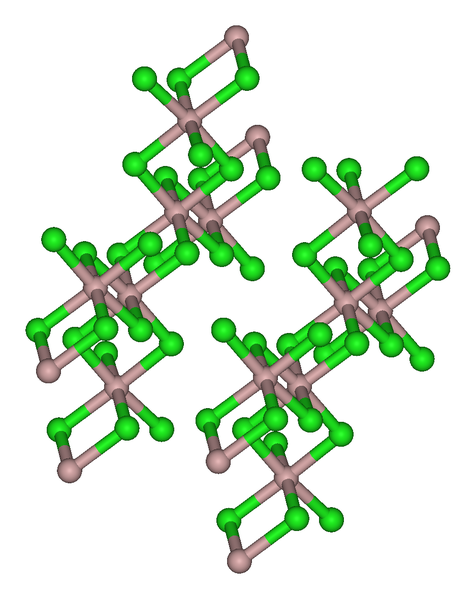
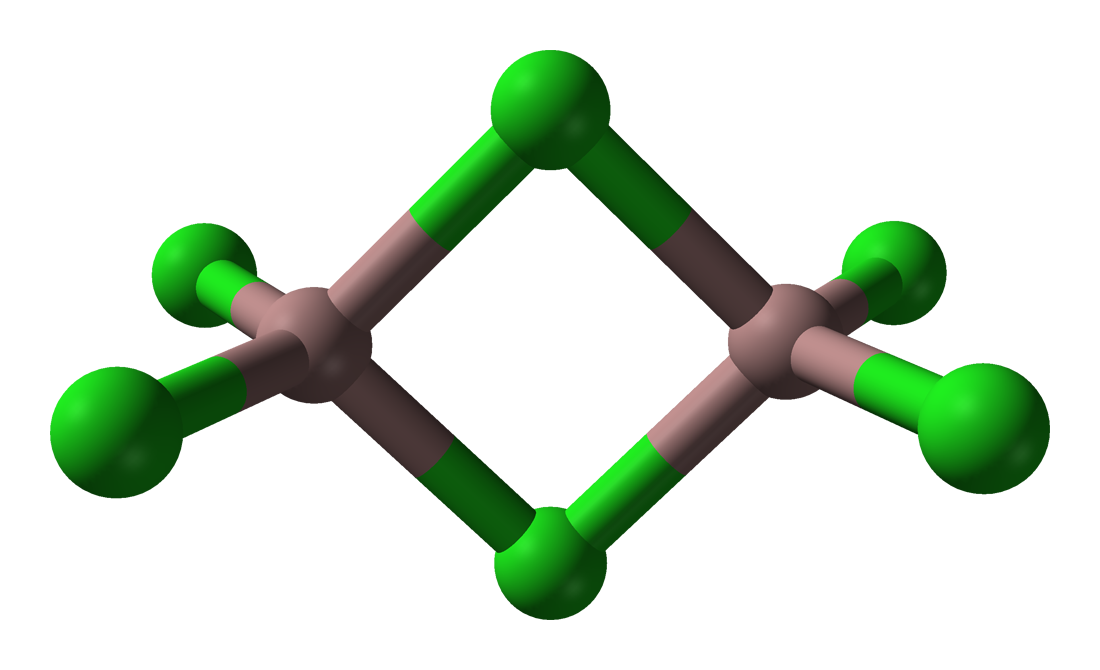
| Compound | M-Xt (Å)a | M-Xb (Å)a | Xt-M-Xt (°)a | Xb-M-Xb (°)a | M-X-M (°) |
| Al2Br6 | 2.21 | 2.33 | 115 | 93 | 87 |
| In2I6 | 2.64 | 2.84 | 125.1 | 93.7 | 86.3 |
Synthesis
Boron trifluoride (BF3) is manufactured commercially by the reaction of boron oxides with hydrogen fluoride, (6.14.1). The HF is produced in-situ from sulfuric acid and fluorite (CaF2). On smaller scales, BF3 is prepared by the thermal decomposition of diazonium salts, (6.14.2).
\[ \text{B}_2\text{O}_3 \text{ + 6 HF} \rightarrow \text{2 BF}_3 \text{ + 3 H}_2\text{O}\]
\[ \text{PhN}_2\text{[BF}_4\text{]} \rightarrow \text{PhF + BF}_3 \text{ + N}_2 \]
Boron trichloride is also made from boron oxide, but in the presence of carbon, (6.14.3).
\[ \text{B}_2\text{O}_3 \text{ + 3 C + 3 Cl}_2 \rightarrow \text{2 BCl}_3 \text{ + 3 CO}\]
Many of the trihalides are readily prepared by either the direct reaction of the metal with the appropriate halogen, (6.14.4) - (6.14.6), or the acid, (6.14.7) and (6.14.8). Thallium tribromide can be prepared in CH3CN by treating a solution of the monobromide with bromine gas, (6.14.9).
\[ \text{2 Al + 3 Cl}_2 \rightarrow \text{2 AlCl}_3\]
\[ \text{2 Al + 3 Br}_2 \rightarrow \text{2 AlBr}_3\]
\[ \text{2 Al + 3 I}_2 \rightarrow \text{2 AlCl}_3\]
\[ \text{2 Al + 6 HCl} \rightarrow \text{2 AlCl}_3 \text{ + 3 H}_2\]
\[ \text{2 Al + 6 HBr} \rightarrow \text{2 AlBr}_3 \text{ + 3 H}_2\]
\[ \text{TlBr + Br}_2 \rightarrow \text{TlBr}_2 \]
Reactivity
The reaction chemistry of the Group 13 trihalides tends to fall into two categories:
- Lewis acid-base complex formation.
- Hydrolysis.
There are, however, a number of reactions involving halide exchange reactions. Aluminum tribromide reacts with carbon tetrachloride at 100 °C to form carbon tetrabromide, (6.14.10), and with phosgene yields carbonyl bromide and aluminum chlorobromide, (6.14.11).
\[ \text{4 AlBr}_3 \text{ + 3 CCl}_4 \rightarrow \text{4 AlCl}_3 \text{ + 3 CBr}_4 \]
\[ \text{AlBr}_3 \text{ + COCl}_2 \rightarrow \text{COBr}_2 \text{ + AlCl}_2\text{Br}\]
Group 13 halides are used as synthons for their organometallic derivatives, (6.14.12) and (6.14.13).
\[ \text{MX}_3 \text{ + 3 RMgX} \rightarrow \text{MR}_3 \text{ + MgX}_2\]
\[ \text{2 MR}_3 \text{ + MX}_3 \rightarrow \text{3 MXR}_2\]
Lewis acid-base complexes
All of the trihalides are strong Lewis acids, and as such react with Lewis base compounds to form Lewis acid-base complexes, (6.14.12). The extent of the equilibrium is dependant on the Lewis acidity of the trihalide and the basicity of the Lewis base. For example, with BCl3, oxygen donor ligands result in approximately 50:50 ratio of BCl3 and BCl3L, while for nitrogen donor ligands the equilibrium is shifted to the formation of the complex.
\[ \text{MX}_3 \text{ + L} \rightleftharpoons \text{X}_3\text{M-L}\]
The general structure of the Lewis acid-base complexes is such that the Group 13 element is close to tetrahedral (Figure \(\PageIndex{4}\)). However, for aluminum and the heavier Group 13 elements, more than one ligand can coordinate (Figure \(\PageIndex{5}\)) up to a maximum of six.
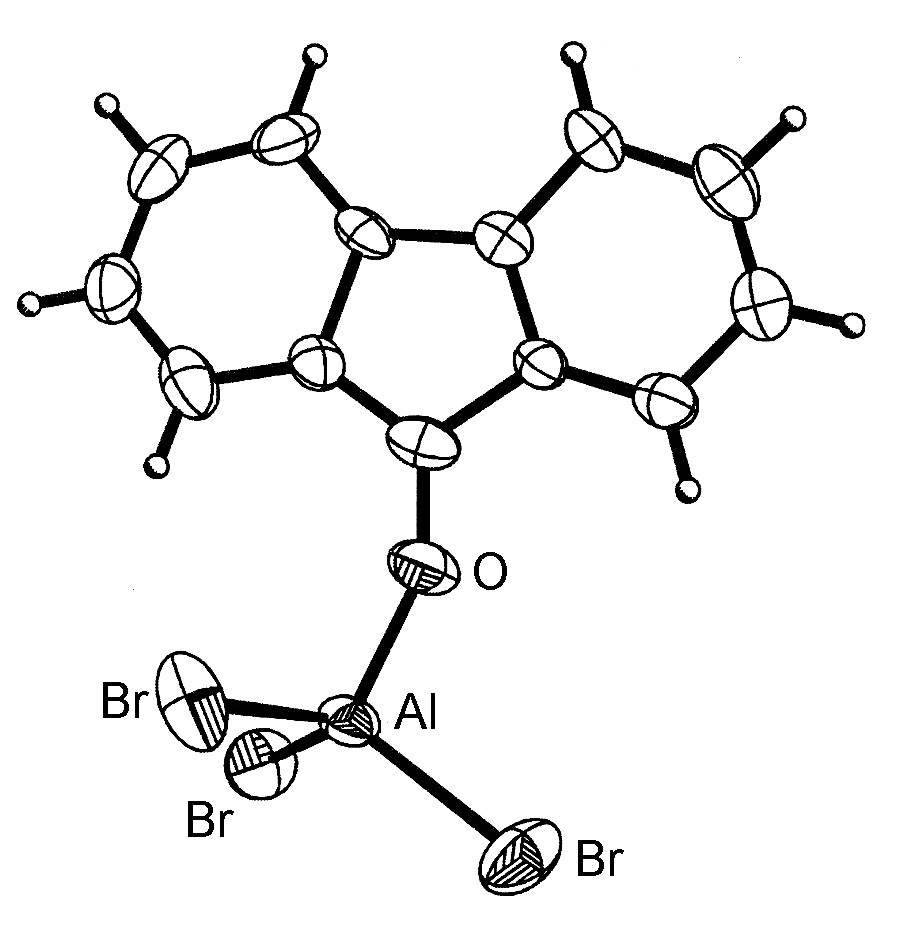
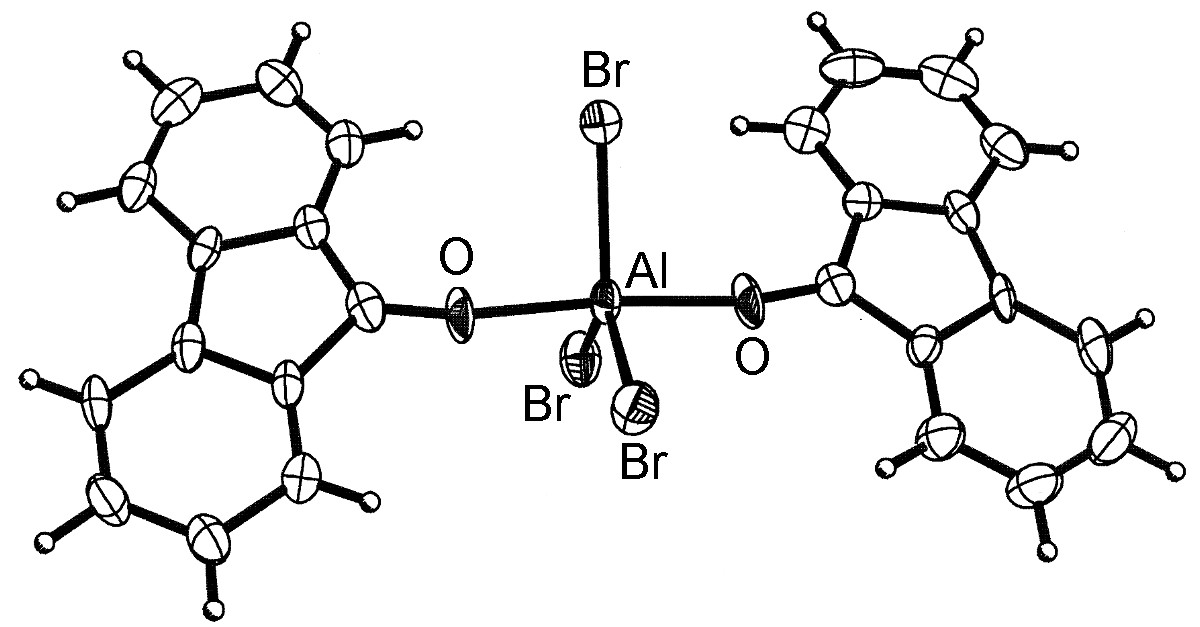
It should be noted that the dimeric form of MX3 (Figure \(\PageIndex{3}\)) can be thought of as mutual Lewis acid-base complexes, in which a Lewis basic lone pair of a halide on one MX3 unit donates to the Lewis acidic metal on another MX3 unit.
Hydrolysis
Generally the fluorides are insoluble in water while the heavier halides are more soluble. However, BF3, BCl3, and BBr3 all decompose in the presence of water, (6.14.13). In the case of the fluoride, the HF formed reacts with BF3 to form fluoroboric acid, (6.14.14). However, there is also a minor equilibrium (2-3%) resulting in the formation of the BF3 complex of OH- and H3O+, (6.14.15).
\[ \text{BX}_3\text{ + H}_2\text{O} \rightarrow \text{B(OH)}_3\text{ + 3 HX}\]
\[ \text{HF + BF}_3 \rightarrow \text{HBF}_4\]
\[ \text{BF}_4 \text{ + H}_3\text{O} \rightleftharpoons \text{F}_3\text{B-OH}_2 \rightleftharpoons \text{[BF}_3\text{OH][BF}_3\text{(OH}_3\text{)]}\]
While the boron compounds (and AlBr3) decompose even in moist air, AlCl3 reacts more slowly to make aluminum chlorohydrate (ACH) which has the general formula AlnCl3n-m(OH)m. While ACH has been proposed to exist as a number of cluster species, it is actually a range of nanoparticles.
ACH is also known as polyaluminum chloride (PAC). The latter name is often used in water purification, where ACH is preferred over alum derivatives (Al2(SO4)3). The combination of ACH and a high molecular weight quaternized ammonium polymer (e.g., dially dimethyl ammonium chloride (DADMAC)), has been known as an effective combination as a flocculant in water treatment process to remove dissolved organic matter and colloidal particles present in suspension.
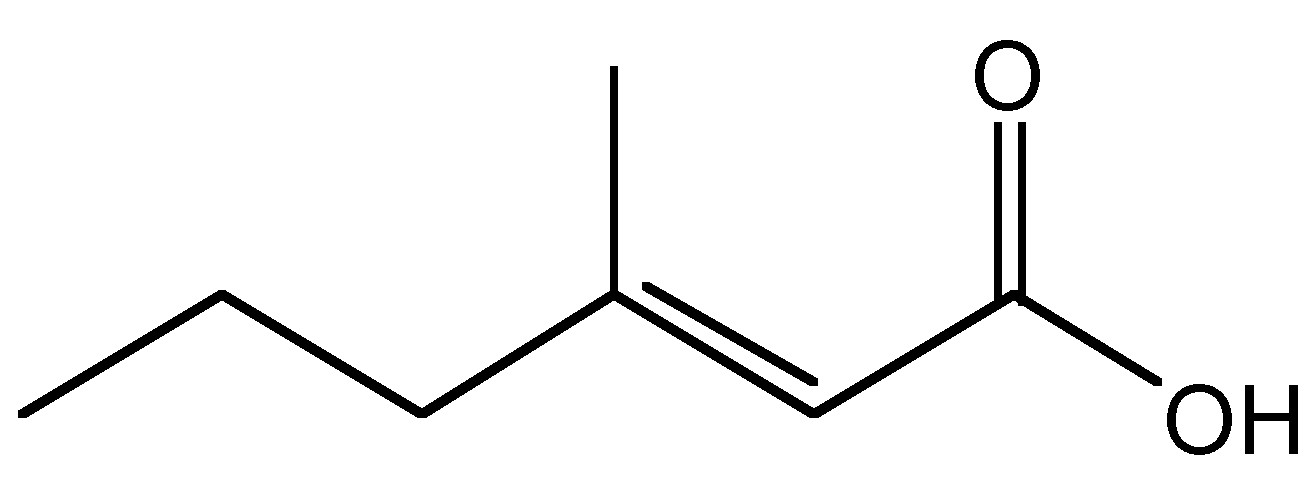
Aluminum chlorohydrate (ACH) and aluminum-zirconium compounds, are frequently used as the active ingredient in antiperspirants. The mode of action of most aluminum-based compounds involves the dramatic change in the particle size from nano to micro as a function of pH and electrolyte changes on the skin (as compared to the antiperspirant stick or suspension) and hence forming a gel plug in the duct of the sweat gland. The plugs prevent the gland from excreting liquid and are removed over time by the natural sloughing of the skin. A further mechanism of action involves the absorption of 3-methyl-2-hexenoic acid (Figure \(\PageIndex{6}\)). Human perspiration is odorless until bacteria ferment it. Bacteria thrive in hot, humid environments such as the underarm. When adult armpits are washed with alkaline pH soaps, the skin loses its acid mantel (pH = 4.5 - 6), raising the skin pH and disrupting the skin barrier. The bacteria thrive in the basic environment, and feed on the sweat from the apocrine glands and on dead skin and hair cells, releasing 3-methyl-2-hexenoic acid, which is the primary cause of body odor. As with all carboxylic acids, 3-methyl-2-hexenoic acid, reacts in a facile manner with the surface of the alumina nanoparticles. Aluminum chloride salts also have a slight astringent effect on the pores; causing them to contract, further preventing sweat from reaching the surface of the skin.
Boron trihalides: a special case
The three lighter boron trihalides, BX3 (X = F, Cl, Br) form stable adducts with common Lewis bases. Their relative Lewis acidities can be evaluated in terms of the relative exothermicity of the adduct-forming reaction:
\[ \text{BF}_3 \text{ < BCl}_3 \text{ < BBr}_3\text{(strongest Lewis acid)}\]
This trend is opposite to that expected based upon the electronegativity of the halogens. The best explanation of this trend takes into account the extent of π-donation that occurs between the filled lone pair orbital on the halogens and the empty p-orbital on the planar boron (Figure \(\PageIndex{7}\)). As such, the greater the π-bonding the more stable the planar BX3 configuration as opposed to the pyramidalization of the BX3 moiety upon formation of a Lewis acid-base complex, (6.4.12).
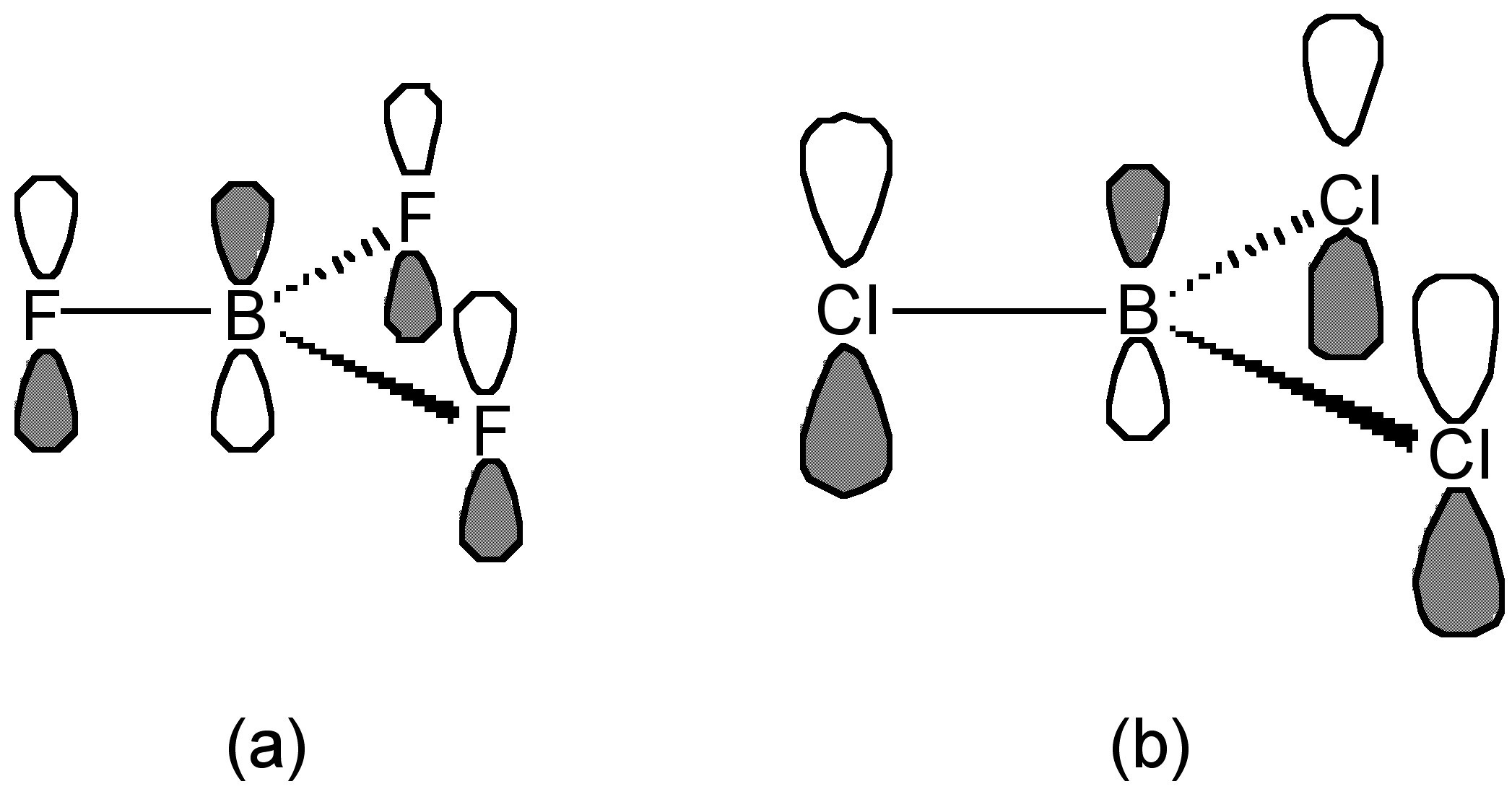
The criteria for evaluating the relative strength of π-bonding are not clear, however, one suggestion is that the F atom is small compared to the Cl atom, and the lone pair electron in pz of F is readily and easily donated and overlapped to empty pz orbital of boron (Figure \(\PageIndex{7}\)a). In contrast, the overlap for the large (diffuse) p-orbitals on the chlorine is poorer (Figure \(\PageIndex{7}\)b). As a result, the π-donation of F is greater than that of Cl. Interestingly, as may be seen from Table \(\PageIndex{4}\), any difference in B-X bond length does not follow the expected trend associated with shortening of the B-X bond with stronger π-bonding. In fact the B-Br distance is the most shortened as compared to that expected from the covalent radii (Table \(\PageIndex{4}\)).
| Compound | B-X (Å) | X covalent radius (Å) | Sum of covalent radii (Å)a | Δ (Å) |
| BF3 | 1.313 | 0.57(3) | 1.41 | 0.097 |
| BCl3 | 1.75 | 1.02(4) | 1.86 | 0.11 |
| BBr3 | 1.898 | 1.20(3) | 2.04 | 0.142 |
| BI3 | 2.125 | 1.39(3) | 2.23 | 0.105 |
At the simplest level the requirements for bonding to occur based upon the molecular or atomic orbital are:
- Directional relationship of the orbitals.
- Relative symmetry of the orbitals.
- Relative energy of the orbitals.
- Extent of orbital overlap
In the case of the boron trihalides, the direction (parallel) and symmetry (p-orbitals) are the same, and the only significant difference will be the relative energy of the donor orbitals (i.e., the lone pair on the halogen) and the extent of the overlap. The latter will be dependant on the B-X bond length (the shorter the bond the greater the potential overlap) and the diffusion of the orbitals (the less diffuse the orbitals the better the overlap). Both of these factors will benefit B-F over B-Cl and B-Br. Thus, the extent of potential overlap would follow the order: (6.14.16). Despite these considerations, it is still unclear of the exact details of the rationalization of the low Lewis basicity of BF3 as compared to BCl3 and BBr3.
Anionic halides
The trihalides all form Lewis acid-base complexes with halide anions, (6.14.17), and as such salts of BF4-, AlCl4-, GaCl4-, and InCl4- are common.
\[ \text{MX}_3 \text{ + X}^- \rightarrow \text{MX}_4^-\]
In the case of gallium the Ga2Cl7- anion (Figure \(\PageIndex{8}\)) is formed from the equilibrium:
\[ \text{2 GaCl}_4^- \rightleftharpoons \text{Ga}_2\text{Cl}_7^- \text{ + Cl}^-\]
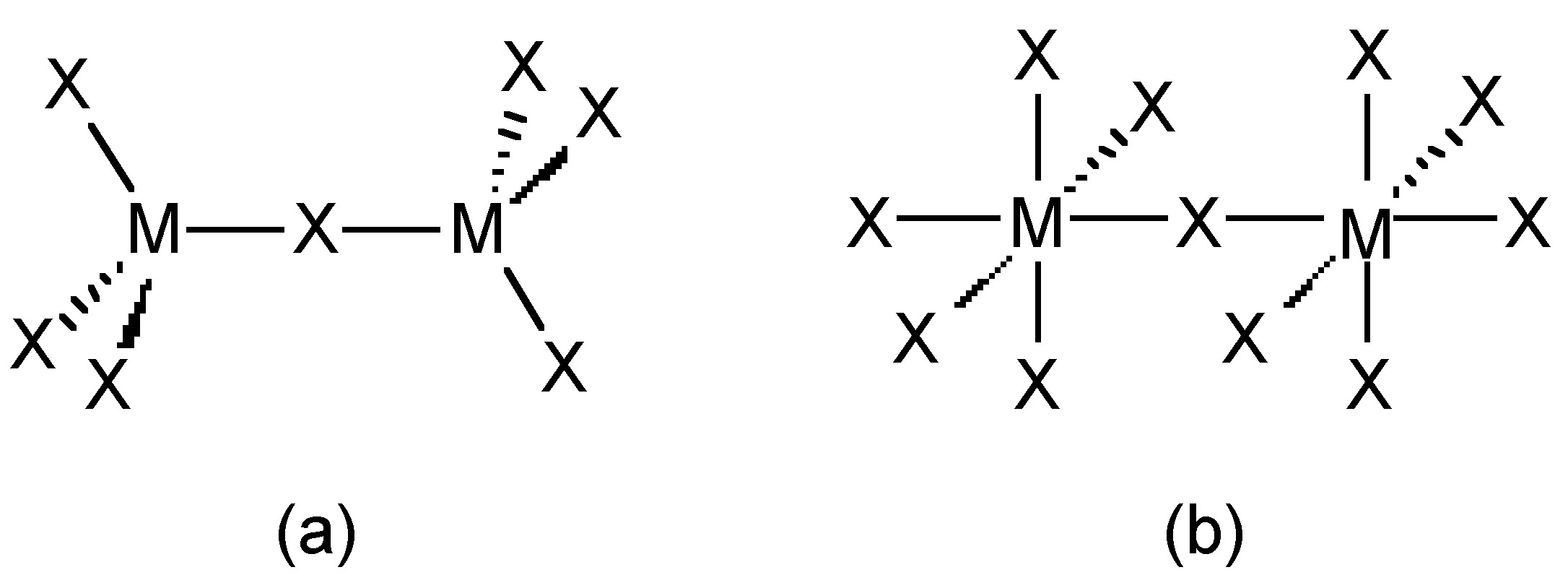
As a consequence of its larger size indium forms a wide range of anionic halides addition compounds with trigonal bipyramidal, square pyramidal, and octahedral coordination geometries. For example, salts of InCl52-, InBr52-, InF63-, InCl63- and InBr63- have all been made. The InCl52- ion has been found to be square pyramidal in the salt [NEt4]2InCl5, but is trigonal bipyramidal in the acetonitrile solvate of [Ph4P]2InCl5. The oligomeric anionic halides In2X7- and In2X93- (X = Cl and Br) contain binuclear anions with tetrahedral and octahedrally coordinated indium atoms, respectively (Figure \(\PageIndex{8}\)).
Low valent halides
Oxidation state +2
Boron forms a series of low oxidation halides containing B-B bonds a formal oxidation state of +2. Passing an electric discharge through BCl3 using mercury electrodes results in the synthesis of B2Cl4, (6.14.19). An alternative route is by the co-condensation of copper as a reducing agent with BCl3, (6.14.20).
\[ \text{2 BCl}_3\text{ + 2 Hg} \rightarrow \text{B}_2\text{Cl}_4 \text{ + Hg}_2\text{Cl}_2\]
\[ \text{2 BCl}_3\text{ + 2 Cu} \rightarrow \text{B}_2\text{Cl}_4 \text{ + 2 CuCl}\]
B2F4 has a planar structure (Figure \(\PageIndex{9}\)) with D2h symmetry, while B2Cl4 has the same basic structure it has a staggered geometry (Figure \(\PageIndex{10}\)). The energy for bond rotation about the B-B bond is very low (5 kJ/mol) that can be compared to ethane (12.5 kJ/mol). The bromide, B2Br4, is also observed to be staggered in the solid state. The staggered conformation is favorable on steric grounds, however, for B2F4 the planar geometry is stabilized by the smaller size of the halide, and more importantly the presence of strong delocalized π-bonding.
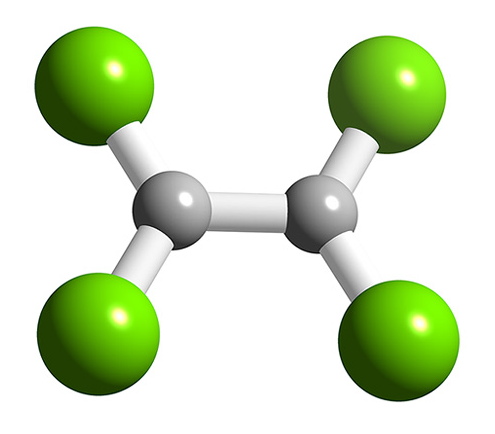
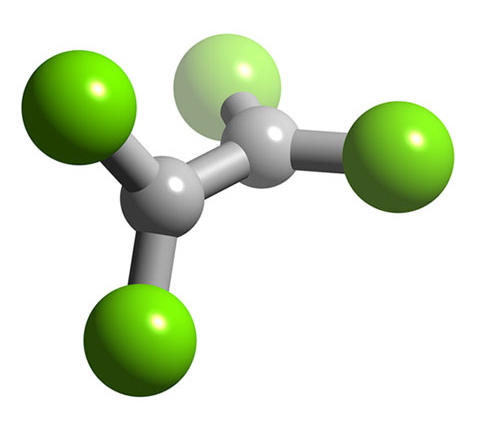
Oxidation state +1
Boron forms a number of halides with cluster structures, BnCln where n = 4 (Figure \(\PageIndex{11}\)), 8, 9, 10, 11, and 12. Each compound is made by the decomposition of B2Cl4. For gallium, none of the monohalides are stable at room temperature, but GaCl and GaBr have been produced in the gas form from the reaction of HX and molten gallium. The stability of thallium(I) as compared to thallium(III) results in the monohalides, TlCl, TlBr, and TlI being stable. Each compound is insoluble in water, and photosensitive.
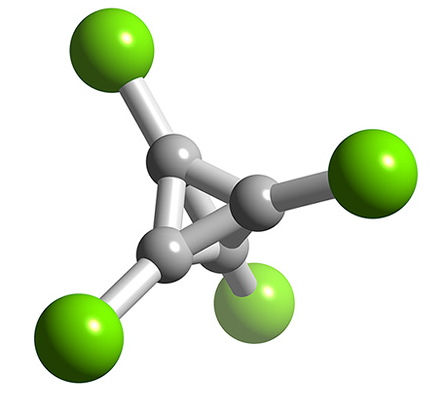
Intermediate halides
The dihalides (MX2) of gallium, indium, and thallium do not actually contain the metal in the +2 oxidation state. Instead they are actually mixed valence compound, i.e., M+[MX4]-. The dihalides of gallium are unstable in the presence of water disproportionating to gallium metal and gallium(III) entities. They are soluble in aromatic solvents, where arene complexes have been isolated and the arene is η6-coordinated to the Ga+ ion. InBr2 and InI2 are greenish and yellow crystalline solids, respectively, which are formulated In(I)[In(III)X4]. TlCl2 and TlBr2 both are of similar formulations.
Ga2X3 (X = Br, I) and In2Br3 are formulated M(I)2[M(II)2X6]. Both anions contain a M-M bond where the metal has a formal oxidation state of +2. The Ga2Br62- anion is eclipsed like the In2Br62- anion, whereas the Ga2I62- anion is isostructural with Si2Cl6 with a staggered conformation. In2Cl3 is colorless and is formulated In(I)3[In(III)Cl6].
Ga3Cl7 contains the Ga2Cl7- ion, which has a structure similar to the dichromate, Cr2O72-, ion with two tetrahedrally coordinated gallium atoms sharing a corner (Figure). The compound can be formulated as gallium(I) heptachlorodigallate(III), Ga(I)[Ga(III)2Cl7].
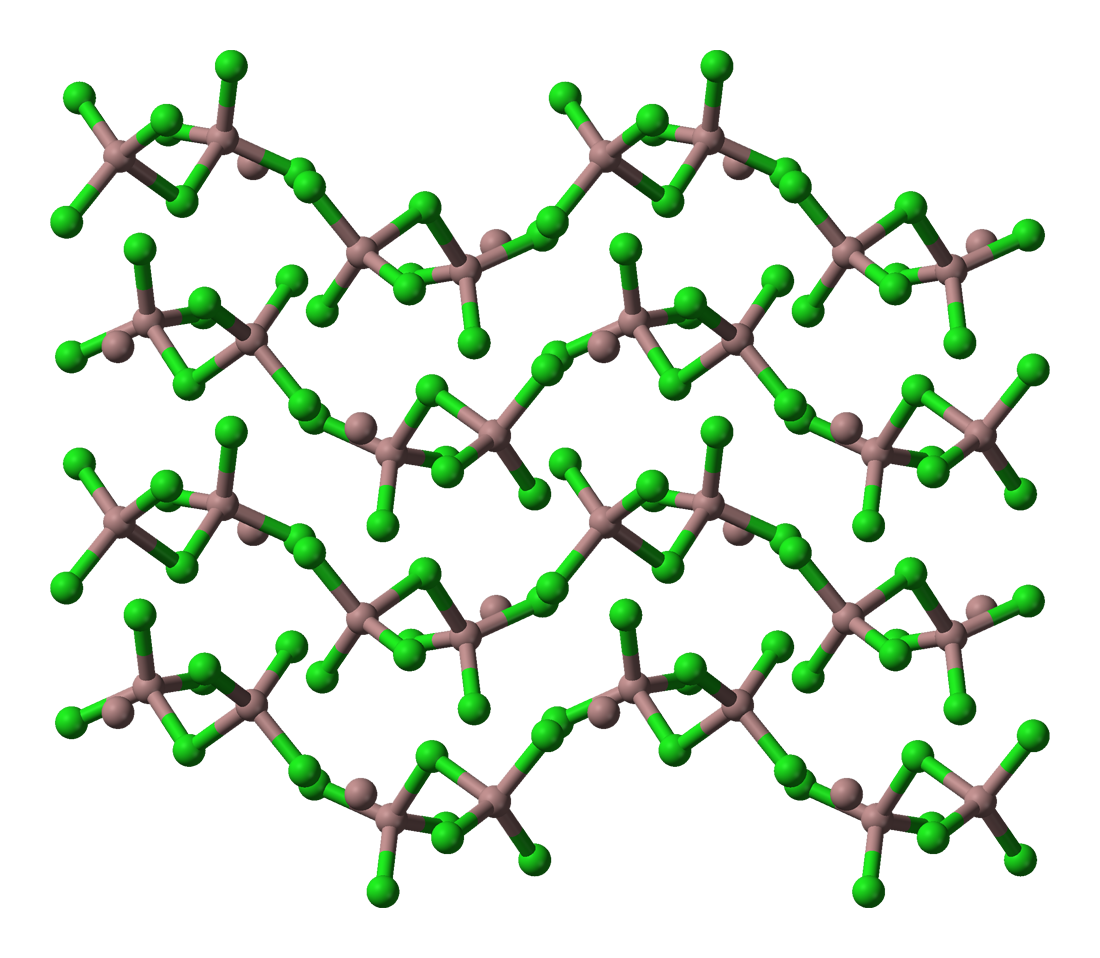
In4Br7 is light sensitive (like TlCl and TlBr) decaying to InBr2 and In metal. It is a mixed salt containing the InBr4- and InBr63- anions balanced by In+ cations. It is formulated In(I)5[In(III)Br4]2[In(III)Br6]. In5Br7 is a pale yellow solid formulated as In(I)3[In(II)2Br6]Br. The In(II)2Br62- anion has an eclipsed ethane like structure with an In-In bond length of 2.70 Å. In5Cl9 is formulated In(I)3[In(III)2Cl9], with the In2Cl92- anion having two 6 coordinate indium atoms with 3 bridging chlorine atoms, face sharing bioctahedra. Finally, In7Cl9 and In7Br9 have a structure formulated as InX6[In(III)X6]X3.
Bibliography
- P. M. Boorman and D. Potts, Can. J. Chem., 1974, 52, 2016.
- A. Borovik and A. R. Barron, J. Am. Chem. Soc., 2002, 124, 3743.
- A. Borovik, S. G. Bott, and A. R. Barron, J. Am. Chem. Soc., 2001, 123, 11219.
- C. S. Branch, S. G. Bott, and A. R. Barron, J. Organomet. Chem., 2003, 666, 23.
- W. M. Brown and M. Trevino, US Patent 5,395,536 (1995).
- S. K. Dentel, CRC Critical Reviews in Environmental Control, 1991, 21, 41.
- D. E. Hassick and J. P. Miknevich, US Patent 4,800,039 (1989).
- M. D. Healy, P. E. Laibinis, P. D. Stupik and A. R. Barron, J. Chem. Soc., Chem. Commun., 1989, 359.
- K. Hedberg and R. Ryan, J. Chem. Phys., 1964, 41, 2214.
- Y. Koide and A. R. Barron, Organometallics, 1995, 14, 4026.
- G. Santiso-Quiñones and I. Krossing, Z. Anorg. Allg. Chem., 2008, 634, 704.

