6.1: The Group 13 Elements
- Page ID
- 212644
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Table \(\PageIndex{1}\) lists the derivation of the names of the Group 13 (IIIA) elements.
| Element | Symbol | Name |
| Boron | B | From the Arabic word buraq or the Persian word burah for the mineral borax |
| Aluminium (Aluminum) | Al | From alum |
| Gallium | Ga | From Named after the Latin word for France (Gaul) Gallia |
| Indium | In | Latin rubidus meaning deepest red |
| Thalium | Tl | From the Latin thallus meaning sprouting green twig |
Table \(\PageIndex{1}\): Derivation of the names of each of the alkali metal elements.
Note
Aluminium is the international spelling standardized by the IUPAC, but in the United States it is more commonly spelled as aluminum.
Discovery
Boron
Borax (a mixture of Na2B4O7.4H2O and Na2B4O7.10H2O) was known for thousands of years. In Tibet it was known by the Sanskrit name of tincal. Borax glazes were used in China in 300 AD, and the writings of the Arabic alchemist Geber appear to mention it in 700 AD. However, it is known that Marco Polo brought some borax glazes back to Italy in the 13th century. In 1600 Agricola in his treatise De Re Metallica reported the use of borax as a flux in metallurgy.


Boron was not recognized as an element until its isolation by Sir Humphry Davy, Joseph Louis Gay-Lussac and Louis Jacques Thénard in 1808 through the reaction of boric acid and potassium. Davy called the element boracium. Jöns Jakob Berzelius identified boron as an element in 1824.




Aluminum
Ancient Greeks and Romans used aluminum salts as dyeing mordants and as astringents for dressing wounds; alum (KAl(SO4)2.12H2O) is still used as a styptic (an antihaemorrhagic agent). In 1808, Sir Humphry Davy identified the existence of a metal base of alum, which he at first termed alumium and later aluminum.
The metal was first produced in 1825 (in an impure form) by Hans Christian Ørsted by the reaction of anhydrous aluminum chloride with potassium amalgam. Friedrich Wöhler repeated the experiments of Ørsted but suggested that Ørsted had only isolated potassium. By the use of potassium (Equation 6.1.1), he is credited with isolating aluminum in 1827. While Wöhler is generally credited with isolating aluminum, Ørsted should also be given credit.
\[ \text{AlCl}_3 \text{ + 3 K} \rightarrow \text{Al + 3 KCl}\]

In 1846 Henri Deville improved Wöhler's method, and described his improvements in particular the use of sodium in place of the expensive potassium
\[ \text{AlCl}_3 \text{ + 3 Na} \rightarrow \text{Al + 3 NaCl}\]

Gallium
The element gallium was predicted, as eka-aluminum, by Mendeleev in 1870, and subsequently discovered by Lecoq de Boisbaudran in 1875; in fact de Boisbaudran had been searching for the missing element for some years, based on his own independent theory. The first experimental indication of gallium came with the observation of two new violet lines in the spark spectrum of a sample deposited on zinc. Within a month of these initial results de Boisbaudran had isolated 1 g of the metal starting from several hundred kilograms of crude zinc blende ore. The new element was named in honor of France (Latin Gallia), and the striking similarity of its physical and chemical properties to those predicted by Mendeleev did much to establish the general acceptance of the periodic Law; indeed, when de Boisbaudran first stated that the density of Ga was 4.7 g cm-3 rather than the predicted 5.9 g/cm3, Mendeleev wrote to him suggesting that he redetermine the value (the correct value is 5.904 g/cm3).


Indium
While testing ores from the mines around Freiberg, Saxony, Ferdinand Reich and Hieronymous Theodor Richter when they dissolved the minerals pyrite, arsenopyrite, galena and sphalerite in hydrochloric acid, and since it was known that ores from that region contained thallium they searched for the green emission lines by spectroscopy. Although the green lines were absent, a blue line was present in the spectrum. As no element was known with a bright blue emission they concluded that a new element was present in the minerals. They named the element with the blue spectral line indium. Richter went on to isolate the metal in 1864.


Thallium
After the publication of their improved method of flame spectroscopy by Robert Bunsen and Gustav Kirchhoff this method became an accepted method to determine the composition of minerals and chemical products. Two chemists, William Crookes and Claude-Auguste Lamy, both started to use the new method and independently employed it in their discovery of thallium.



Crookes was making spectroscopic determinations on selenium compounds deposited in the lead chamber of a sulfuric acid production plant near Tilkerode in the Harz mountains. Using a similar spectrometer to Crookes', Lamy was determining the composition of a selenium-containing substance that was deposited during the production of sulfuric acid from pyrite. Using spectroscopy both researchers both observed a new green line the atomic absorption spectrum and assigned it to a new element. Both set out to isolate the new element. Fortunately for Lamy, he had received his material in larger quantities and thus he was able to isolate sufficient quantities of thallium to determine the properties of several compounds and prepare a small ingot of metallic thallium. At the same time Crookes was able to isolate small quantities of elemental thallium and determine the properties of a few compounds. The claim by both scientists resulted in significant controversy during 1862 and 1863; interestingly this ended when Crookes was elected Fellow of the Royal Society in June 1863.
Abundance
The abundance of the Group 13 elements is given in Table \(\PageIndex{2}\). Aluminum is the most abundant metal in the Earth’s crust and is found in a wide range of minerals. While boron is not as common it is also found in a range of borate minerals. In contrast, gallium, indium, and thallium are found as impurities in other minerals. In particular indium and thallium are found in sulfide or selenide mineral rather than oxides, while gallium is found in both sulfides (ZnS) and oxides (bauxite). Although indium and thallium minerals are known, they are rare: indite (FeIn2S4), lorandite (TlAsS2), crookesite (Cu7TlSe4).
| Element | Terrestrial abundance (ppm) |
| B | 10 (Earth’s crust), 20 (soil), 4 (sea water) |
| Al | 82,000 (Earth’s crust), 100,000 (soil), 5 x 10-4 (sea water) |
| Ga | 18 (Earth’s crust), 28 (soil), 30 x 10-6 (sea water) |
| In | 0.1 (Earth’s crust), 0.01 (soil), 0.1 x 10-6 (sea water) |
| Tl | 0.6 (Earth’s crust), 0.2 (soil), 10 x 10-6 (sea water) |
Isotopes
The naturally abundant isotopes of the Group 13 elements are listed in Table \(\PageIndex{3}\). Thallium has 25 isotopes that have atomic masses that range from 184 to 210. Thallium-204 is the most stable radioisotope, with a half-life of 3.78 years.
| Isotope | Natural abundance (%) |
| Boron-10 | 19.9 |
| Boron-11 | 80.1 |
| Aluminum-27 | 100 |
| Gallium-69 | 60.11 |
| Gallium-71 | 39.89 |
| Indium-113 | 4.3 |
| Indium-115 | 95.7 |
| Thallium-203 | 29.52 |
| Thallium-205 | 70.48 |
The Group 13 elements offer potential as NMR nuclei (Table \(\PageIndex{4}\)). In particular 11B and 27Al show promise for characterization in both solution and solid state.
| Isotope | Spin | Natural abundance (%) | Quadrupole moment (10-30 m2) | NMR frequency (MHz) at a field of 2.3488 T | Reference |
| Boron-10 | 3 | 19.58 | 8.459 | -10.746 | BF3.Et2O |
| Boron-11 | 3/2 | 80.42 | 4.059 | -32.084 | BF3.Et2O |
| Aluminum-27 | 5/2 | 100 | 14.66 | -26.057 | Al(NO3)3 |
| Gallium-69 | 3/2 | 60.4 | 17.1 | -24.003 | Ga(NO3)3 |
| Gallium-71 | 3/2 | 39.6 | 10.7 | -30.495 | Ga(NO3)3 |
| Indium-113 | 9/2 | 4.28 | 79.9 | -21.866 | In(NO3)3 |
| Indium-115 | 9/2 | 95.72 | 81.0 | -21.914 | In(NO3)3 |
Industrial production
Borax is mined as a mixture of Na2B4O7.4H2O and Na2B4O7.10H2O. Acidification gives boric acid, B(OH)3, which can be reduced with sodium amalgam (Na/Hg) to give amorphous boron. Pure boron can be prepared by reducing boron halides (e.g., BF3 and BCl3) with hydrogen at high temperatures. Ultrapure boron, for the use in semiconductor industry, is produced by the decomposition of diborane (B2H6) and then further purified with the zone melting or Czochralski processes.
The only two economic sources for gallium are as byproduct of aluminum and zinc production. Extraction during the Bayer process followed by mercury cell electrolysis and hydrolysis of the amalgam with sodium hydroxide leads to sodium gallate. Electrolysis then gives gallium metal.
The lack of indium mineral deposits and the fact that indium is enriched in sulfides of lead, tin, copper, iron and zinc, makes the zinc production the main source for indium. The indium is leached from slag and dust of zinc production. Up until 1924, there was only about a gram of isolated indium on the planet, however, today worldwide production is currently greater 476 tons per year from mining and a 650 tons per year from recycling. This massive increase in demand is due to applications in LCD displays and solar cell applications.
Aluminum
Due to aluminum’s position as the most abundant metallic element in the Earth's crust (7.5 - 8.1%) and its enormous industrial importance warrants detailed discussion of its industrial production. Aluminum only appears in its elemental form in nature in oxygen-deficient environments such as volcanic mud. Ordinarily, it is found in a variety of oxide minerals.
In comparison to other metals aluminum is difficult to extract from its ores. Unlike iron, aluminum oxides cannot be reduced by carbon, and so purification is only possible on an economic scale using electrolysis. Prior to electrolysis purified aluminum oxide is obtained by refining bauxite in the process of developed by Karl Bayer.

Bauxite, the most important ore of aluminum, contains only 30-50% alumina, Al2O3, the rest being a mixture of silica, iron oxide, and titanium dioxide. Thus, the alumina must be purified before it can be used as the oxide or refined into aluminum metal. In the Bayer process, bauxite is digested in hot (175 °C) sodium hydroxide (NaOH) solution (Figure). This converts the alumina to aluminum hydroxide, Al(OH)3, which dissolves in the hydroxide solution.
\[ \text{Al}_2\text{O}_3 \text{ + 2 OH}^- \text{ + 3 H}_2\text{O} \rightarrow \text{2 [Al(OH)}_4\text{]}^- \]
The other components do not dissolve and are filtered off. The hydroxide solution is cooled, and the dissolved aluminum hydroxide precipitates, which when heated to 1050 °C is calcined into alumina.
\[ \text{2 Al(OH)}_3 \rightarrow \text{Al}_2\text{O}_3 \text{ + 3 H}_2\text{O}\]
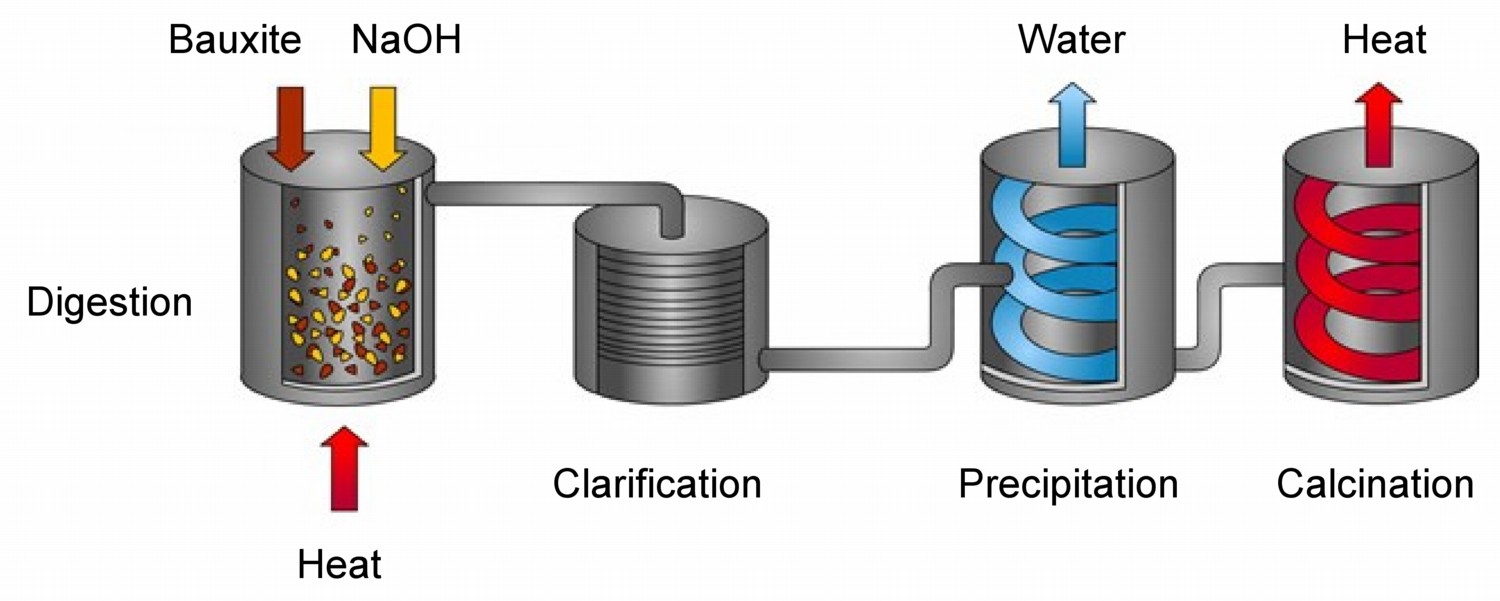
Once a pure alumina is formed, it is dissolved in molten cryolite (Na3AlF6) and reduced to the pure metal at elevated temperatures (950 - 980 °C) using the Hall-Héroult process, developed independently Charles Hall and Paul Héroult.


Both of the electrodes used in the electrolysis of aluminum oxide are carbon. The reaction at the cathode involves the reduction of the Al3+. The aluminum metal then sinks to the bottom and is tapped off, where it is usually cast into large blocks called aluminum billets.
\[\text{Al}^{3+} + \text{3 e}^- \rightarrow \text{Al}\]
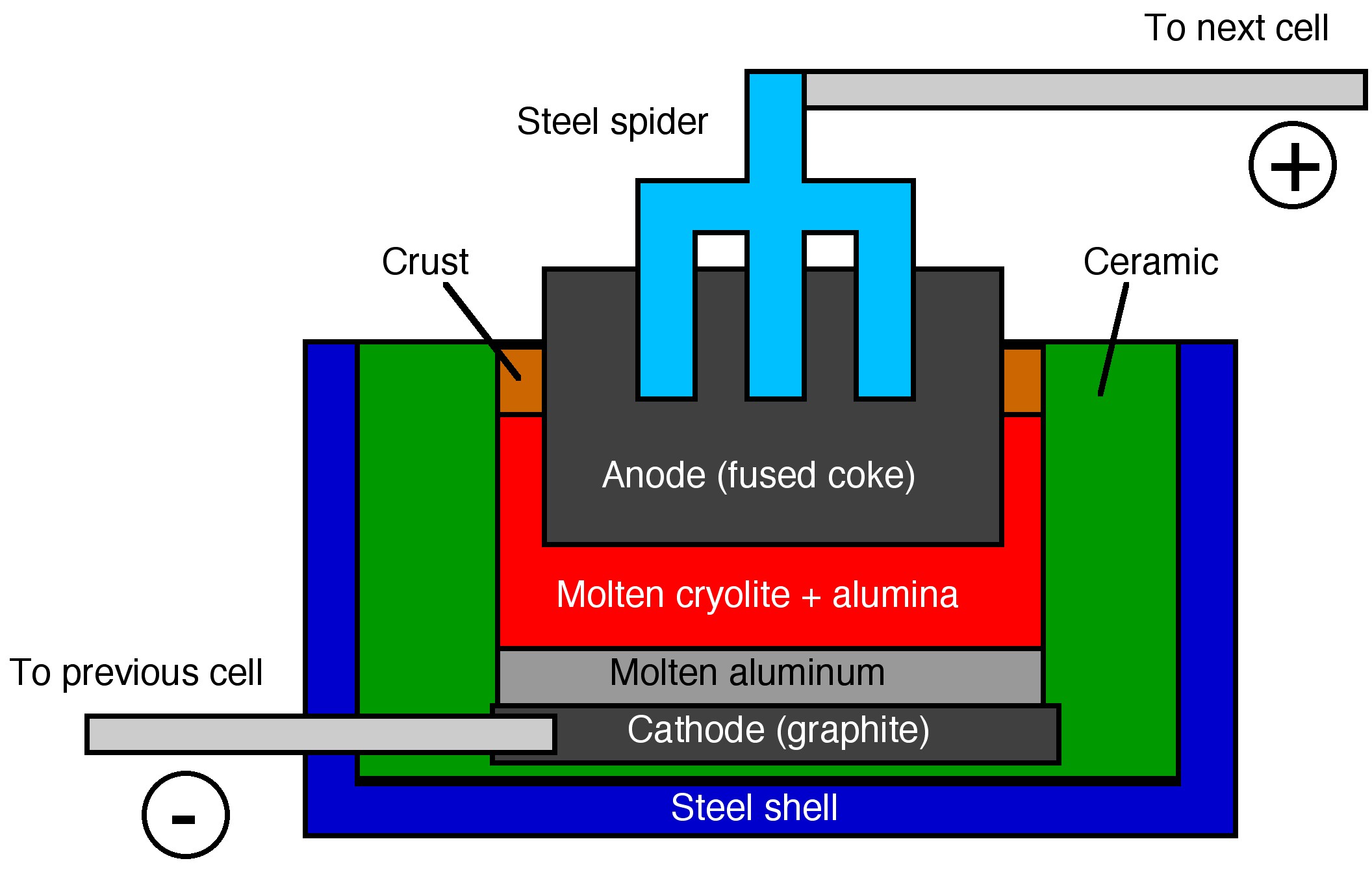
At the anode oxygen is formed, (6.1.6) , where it reacts with the carbon anode is then oxidized to carbon dioxide, (6.1.7). The anodes must be replaced regularly, since they are consumed. While the cathodes are not oxidized they do erode due to electrochemical processes and metal movement.
\[ \text{2 O}_2^- \rightarrow \text{O}_2 + \text{4 e}^-\]
\[ \text{C + O}_2 \rightarrow \text{CO}_2 \]
Although the Hall-Héroult process consumes a lot of energy, alternative processes have always found to be less economically and ecologically viable.
Physical properties
Table summarizes the physical properties of the Group 13 elements. While, aluminum, indium, and thallium have typical metal properties, gallium has the largest liquid range of any element. Boron exists as a molecular compound in the solid state, hence its high melting point.
| Element | Mp (°C) | Bp (°C) | Density (g/cm3) |
| B | 2300 | 3658 | 2.3 |
| Al | 661 | 2467 | 2.7 |
| Ga | 30 | 2403 | 5.9 (solid), 6.1 (liquid) |
| In | 156 | 2080 | 7.3 |
| Tl | 304 | 1457 | 11.9 |
Table \(\PageIndex{5}\): Selected physical properties of the Group 13 elements.
Bibliography
- C. M. Hall. Process of reducing aluminum from its fluoride salts by electrolysis. US Patent 400,664 (1886).
- L. B. Alemany, S. Steuernagel, J.-P. Amoureux, R. L. Callender, and A. R. Barron. Solid State Nuclear Magnetic Resonance, 1999, 14, 1.
- L. B. Alemany, R. L. Callender, A. R. Barron, S. Steuernagel, D. Iuga, and A. P. M. Kentgens, J. Phys. Chem., B., 2000, 104, 11612.
- M. Bishop, N. Shahid, J. Yang, and A. R. Barron, Dalton Trans., 2004, 2621.


