3.4: Organolithium Compounds
- Page ID
- 212626
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One of the major uses of lithium is in the synthesis of organolithium compounds, RLi. They have great importance and utility in industry and chemical research. Their reactivity resembles that of Grignard reagents, but they are generally more reactive.
Synthesis
The best general method for RLi synthesis involves the reaction of an alkyl or aryl chloride with lithium metal in benzene or an aliphatic hydrocarbon (e.g., hexane), (3.4.1).
\[RCl + 2 Li \rightarrow RLi + LiCl \]
While it is possible to use diethyl ether (Et2O), the solvent slowly attack the resultant alkyl lithium compound, (3.4.2).
\[Et_2O + ^nBuLi \rightarrow EtOLi + H_2C\text{=}CH_2 + ^nBuH\]
Metal-hydrogen exchange, (3.4.3), metal-halogen exchange, (3.19), and metal-metal exchange can also be used, (3.4.4).
\[RH + R'Li \rightarrow R'H + RLi \]

\[ 2 Li + R_2Hg \rightarrow 2 RLi + Hg\]
All organolithium compounds are produced as solutions and are hence used in synthetic protocols by volume of solution. It is therefore important to know the exact concentration of RLi in solution. The simplest approach to quantify the amount of organolithium is to react a known volume with water, (3.4.5), and then titrate (with acid) the resultant base that is formed.
\[RLi + H_2O \rightarrow LiOH + RH \]
However, while the concentration of freshly prepared samples of organolithium reagents can the theoretically measured in this way, real samples always contain some amount of LiOH or other bases. A simple titration inevitably results in an over estimation of the organolithium reagent. To overcome this a double titration method is used.
Gillman double titration method
The careful addition of a known volume of an organolithium reagent solution (between 0.5 and 1.5 mL) to an excess of water yields a solution of LiOH that can be titrated with a standardized solution of hydrochloric acid, using phenolphthalein as the indicator. The presence of any LiOH in the original organolithium solution will be incorporated into this titration, and thus the result will be a measure of the total base content in the solution, i.e., (3.4.6).
\[ \text{Total base content} = \text{LiOH formed from the reaction of RLi with H}_2\text{O} + \text{LiOH present as impurity in the RLi solution} \]
In order to determine the amount of LiOH present as impurity in the organolithium solution it is necessary to react the RLi without the formation of base, then titrate the resulting solution. To do this, an aliquot (the same amount as used before) of the organolithium is reacted slowly with 1,2-dibromoethane (BrCH2CH2Br) dissolved in dry diethyl ether (Et2O). After 5 min of stirring, the solution is diluted with an excess of water and then titrated with a standardized solution of hydrochloric acid, again using phenolphthalein as the indicator. The dierence of the two titrations gives the exact concentration of the organolithium.
Example \(\PageIndex{1}\)
An aliquot of nBuLi in hexanes (0.50 mL) was added to degassed water (20 mL). After any visible reaction had ceased, a few drops of a phenolphthalein solution in water/methanol are added resulting in a pink color indicative of a basic pH. The resulting mixture is titrated with standardized hydrochloric acid ([HCl] = 0.1034 N) until complete disappearance of the pink color (7.90 mL).
A second aliquot of nBuLi in hexanes (0.50 mL) is added to 1,2-dibromoethane (0.20 mL, Et2O). After 5 min of stirring, the mixture was diluted with water (20 mL) and after addition of the phenolphthalein indicator titrated (with vigorous stirring due to the biphasic nature of the system) with standardized hydrochloric acid ([HCl] = 0.1034 N) until complete disappearance of the pink color (0.25 mL).
The concentration of nBuLi is calculated as follows:
Step 1. \[ \text{[total base]} = \dfrac{\text{volume HCl } \times \text{ [HCl]}}{\text{volume } ^n \text{BuLi}} = \dfrac{7.90 \times 0.1034}{0.50} = 1.633 \]
Step 2. \[ \text{[residual base]} =\dfrac{\text{volume HC l} \times \text{ [HCl]}}{\text{volume } ^n \text{BuLi}} = \dfrac{0.25 \times 0.1034}{0.50} = 0.013 \]
Step 3. \[ \text{[}^n\text{BuLi]} = \text{[total base]} - \text{[residual base]} = 1.633 - 0.013 = 1.620M\]
Properties
Alkyl lithium compounds are either low melting solids or liquids, and often with high volatility (depending on the substituent) due to the covalent nature of the bonding. They are soluble in aliphatics, aromatics, and ethers. However, while the reaction with ethers is generally slow, (3.17), alkyl lithium compounds can polymerize tetrahydrofuran (THF).
Organolithium compounds react rapidly with air and water (both vapor and liquid). The reaction with water is the basis of the Gillman double titration method for determining the concentration of organolithium reagents in solution.
Structure
The structure of organolithium compounds is dominated by their highly oligomeric nature as a result of 3-center 2-electron bridging bonds. In all cases the extent of oligomerization is dependant on the identity of the alkyl (or aryl) group. The alkyl-bridged bond is similar to those found for beryllium and aluminum compounds.
In the vapor phase any particular organolithium derivative show a range of oligomeric structures. For example, the mass spectrum of EtLi shows ions associated with both tetramers (e.g., [Et3Li4]+) and hexamers (e.g., [Et5Li6]+). The structures of the different oligomers have been predicted by molecular orbital calculations (Figure \(\PageIndex{3}\).20).
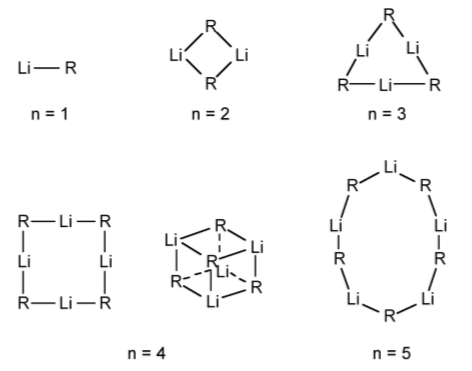
Solution molecular weight measurements indicate the oligomerization is present (in the absence of a coordinating ligand such as Et2O or an amine). The extent of oligomerization depends on the steric bulk of the alkyl group (Table \(\PageIndex{3}\).16). Oligomerization and solution structures have also been investigated by 7Li and 13C NMR spectroscopy.
| R | [RLi]n | R | [RLi]n |
|---|---|---|---|
| Me | 4 | Et | 6 |
| nBu | 6 | tBu | 4 |
There are a large number of X-ray crystallographically determined structures for organolithium derivatives. The archetypal example is MeLi, which exists as a tetramer in the solid state (Figure \(\PageIndex{3}\).21). The lithium atoms are arranged as a tetrahedron and the carbon atoms are positioned on the center of the facial planes, i.e., the carbon is equidistant from each of the lithium atoms. In contrast, EtLi has a similar tetrahedral structure, but the α-carbon of the ethyl groups are asymmetrically arranged such that it is closer to one lithium atom than the other two.


