2.5: The Proton
- Page ID
- 212620
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The proton, H+, is the name given to hydrogen in the +1 oxidation state.
Gas phase
The proton can be formed from the photolysis of atomic hydrogen in the vapor phase at low pressure.
\[H^._{(g)} + h\nu \rightarrow H^+_{(g)} + e^-_{(g)}\]
The proton is more reactive than the hydrogen atom because of its high charge density. In addition, the proton's small ionic radius, 1.5 x 10-15 cm, means that it can get close to other atoms and hence form strong bonds.
\[H^+_{(g)} + NR_3 \rightarrow HNR^+_{3(g)}\]
The strength of the bonding interaction is such that it is very hard to measure directly. Instead the relative bond strength between the proton and an appropriate base, B1, is measured in the presence of a competing base, B2.
\[B_1H^+_{(g)} + B_{2(g)} \leftrightharpoons B_2H^+_{(g)} + B_{2(g)}\]
In measuring the exchange reaction, the relative proton affinity of B1 and B2 is measured. This is also known as the gas phase acidity, and as such it is a measure of the inherent acidity of a species X-H because it obviates any solvent effects.
Liquid and solution
The high reactivity of the proton means that it does not exist free in solution. There are however many H+ containing species. These are generally classified as acids.
\[ B_1H^+_{(sol)} + B_{2(sol)} \leftrightharpoons B_2H^+_{(sol)} + B_{1(sol)} \\ \text{acid base acid base}\]
The reaction between the acid and the base is a proton transfer reaction. While the proton travels from B1 to B2, it is never free in solution. Instead a bridged transition state or intermediate is formed, B1...H+...B2.
Acidity and pH
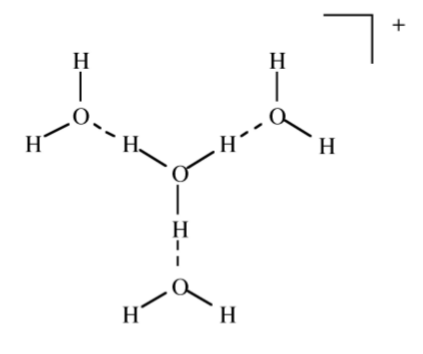
The most common solvent for H+ is water. The acid form is usually defined as the hydronium ion or H3O+, (2.5.5). The terms oxonium, hydroxonium and oxidanium are also used for the H3O+. Although we commonly use H3O+ it is known from spectroscopy that larger complexes are formed such as H9O4+ (Figure \(\PageIndex{2}\).10).
\[H_2O + H^+ \rightarrow H_3O^+ \]
Acids and bases have been characterized in a number of different ways. In 1680 Robert Boyle (Figure \(\PageIndex{2}\).11) defined an acid as a compound that dissolved many other compounds, had a sour taste, and reacted with alkali (base).

Boyle's simple observational description was rationalized by Danish physical chemist Johannes Brønsted (Figure \(\PageIndex{2}\).12). Brønsted proposed that acids are proton donors, and bases are proton acceptors. An acid-base reaction is one in which a proton is transferred from a proton donor (acid) to a proton acceptor (base). Based upon Brønsted's proposal simple acids contain an ionizable proton. Examples of simple acids include neutral molecules (HCl, H2SO4), anions (HSO4-, H2PO4-), and cations (NH4+). The most common Brønsted bases include metal hydroxides (MOH).

Brønsted noted that when an acid donates a proton it forms a conjugate base. The following are examples of an acid and its conjugate base.
\[ H_2O \space\space \rightarrow H^+ + OH^- \\ \text{acid conjugate base}\]
\[ H_2SO_4 \space\space \rightarrow H^+ + HSO^-_4 \\ \text{acid conjugate base}\]
\[NH^+_4 \space\space\space\space \rightarrow H^+ + NH_3 \\ \text{acid conjugate base}\]
Exercises
Exercise \(\PageIndex{1}\)\
What is the conjugate base of HCl?
- Answer
-
Cl-
Exercise \(\PageIndex{2}\)\
What is the conjugate base of HSO4-
- Answer
-
SO42-
Exercise \(\PageIndex{3}\)\
What is the conjugate base of [Al(H2O)6]3+
- Answer
-
[Al(H2O)5(OH)]2+
The same occurs when a base accepts a proton it forms a conjugate acid. The following are examples of a base and its conjugate acid.
\[ H_2O \space\space + \space\space H^+ \rightarrow H_3O^+ \\ \text{base conjugate acid}\]
\[HCO^-_3 \space\space + \space\space H^+ \rightarrow H_2CO_3 \\ \text{base conjugate acid}\]
\[F^- \space\space + \space\space H^+ \rightarrow HF \\ \text{base conjugate acid}\]
Exercises
Exercise \(\PageIndex{4}\)\
What is the conjugate acid of NH3?
- Answer
-
NH4+
Exercise \(\PageIndex{5}\)\
What is the conjugate acid of S2-?
- Answer
-
HS-
Exercise \(\PageIndex{6}\)\
What is the conjugate acid of CO32-?
- Answer
-
HCO3-
Thus, the reaction between an acid and a base results in the formation of the appropriate conjugate base and conjugate acid.
\[acid_1 + base_1 \leftrightharpoons acid_2 + base_2 \]
A specific example is as follows:
\[HNO_3 + NH_3 \leftrightharpoons NH^+_4 + NO^-_3 \\ acid_1 \space\space\space\space base_1 \space\space\space\space\space\space\space acid_2 \space\space\space\space base_2\]
Exercise \(\PageIndex{7}\)
What is the conjugate acid and base formed from the reaction of NH4+ with S2+?
- Answer
-
\[NH_4^+ + S^{2-} \leftrightharpoons HS^- + NH_3 \\ acid_1 \space\space\space\space base_1 \space\space\space\space\space\space\space acid_2 \space\space\space\space base_2\]
In the equilibrium reactions shown in (2.12) and (2.13) there is a competition between the two bases for the proton. As would be expected the strongest base wins.
When a strong acid is added to (dissolved in) water it will react with the water as a base:
\[HCl + H_2O \leftrightharpoons H_3O^+ + Cl^- \\ acid \space\space\space\space base \space\space\space\space\space\space\space acid \space\space\space\space base\]
In contrast, when a strong base is added to (dissolved in) water it will react with the water as an acid:
\[ H_2O + NH_3 \leftrightharpoons NH_4^+ + OH^- \\ acid \space\space\space\space base \space\space\space\space\space\space\space acid \space\space\space\space base\]
pH a measure of acidity
The acidity of a water (aqueous) solution depends on the concentration of the hydronium ion, i.e., [H3O+]. The acidity of a solution is therefore the ability of the solution to donate a proton to a base. The acidity or pH of a solution is defined as:
\[pH = -log [H_3O^+_a] \]
It is important to note that the value is the activity of H3O+ and not the concentration.
Note
Activity is a measure of the eective concentration of a species in a mixture. The dierence between activity and other measures of composition such as concentration arises because molecules in non-ideal gases or solutions interact with each other, either to attract or to repel each other.
The activity of the H3O+ ion can be measured by
(a) A gas electrode
(b) Acid-base indicators
Proton Transfer Reactions
The proton transfer reaction is one of the simplest reactions in chemistry. It involves no electrons and low mass transfer/change, giving it a low energy of activation. For proton transfer between O-H or N-H groups and their associated bases the reaction is very fast. The proton transfer occurs across a hydrogen-bonded pathway during which the proton is never free.


