5.1: Prelude to Coordination Chemistry and Crystal Field Theory
- Page ID
- 189556
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Coordination compounds (or complexes) are molecules and extended solids that contain bonds between a transition metal ion and one or more ligands. In forming these coordinate covalent bonds, the metal ions act as Lewis acids and the ligands act as Lewis bases. Typically, the ligand has a lone pair of electrons, and the bond is formed by overlap of the molecular orbital containing this electron pair with the d-orbitals of the metal ion. Ligands that are commonly found in coordination complexes are neutral molecules (H2O, NH3, organic bases such as pyridine, CO, NO, H2, ethylene, and phosphines PR3) and anions (halides, CN-, SCN-, cyclopentadienide (C5H5-), H-, etc.). The resulting complexes can be cationic (e.g., [Cu(NH3)4]2+), neutral ([Pt(NH3)2Cl2]) or anionic ([Fe(CN)6]4-). As we will see below, ligands that have weak or negligible strength as Brønsted bases (for example, CO, CN-, H2O, and Cl-) can still be potent Lewis bases in forming transition metal complexes.
With ligands that are Lewis bases, coordinate covalent bonds (also called dative bonds) are typically drawn as lines, or sometimes as arrows to indicate that the electron pair "belongs" to the ligand X:
\(M \leftarrow :X\)
In counting electrons on the metal (described below), the convention is to assign both electrons in the dative bond to the ligand, although in reality the bonds are typically polar covalent and electrons are shared between the metal and the ligand.
|
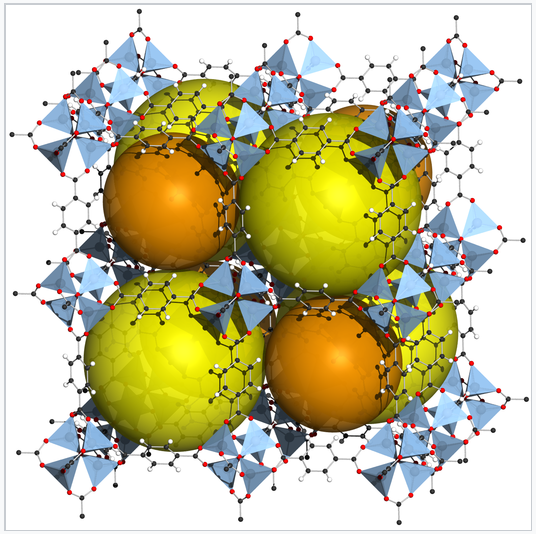
Zn4O(BDC)3, also called MOF-5, is a metal-organic framework in which 1,4-benzenedicarboxylate (BDC) anions bridge between cationic Zn4O clusters.[1] The rigid framework contains large voids, represented by orange spheres. MOFs can be made from many different transition metal ions and bridging ligands, and are being developed for practical applications in storing gases, especially H2 and CO2. MOF-5 has a volumetric storage density of 66 g H2/L, close to that of liquid H2. |
When writing out the formulas of coordination compounds, we use square brackets [...] around the metal ions and ligands that are directly bonded to each other. Thus the compound [Co(NH3)5Cl]Cl2 contains octahedral [Co(NH3)5Cl]2+ ions, in which five ammonia molecules and one chloride ion are directly bonded to the metal, and two Cl- anions that are not coordinated to the metal.
History
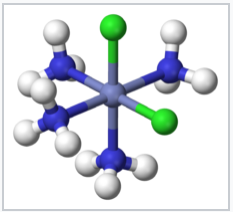
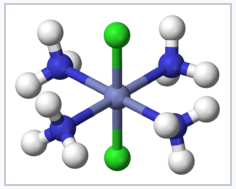
Coordination compounds have been known for centuries, but their structures were initially not understood. For example, Prussian Blue, which has an empirical formula Fe7(CN)18•xH2O, is an insoluble, deep blue solid that has been used as a pigment since its accidental discovery by Diesbach in 1704. Prussian Blue actually contains Fe3+ cations and [Fe(CN)6]4- anions, and a more descriptive formulation is (Fe3+)4([Fe(CN)6]4-)3•xH2O. Simpler compounds such as the ammonia complex of Co3+ were known to chemists but did not fit the expected behavior of ionic solids. For example, cobalt(III)hexammine chloride, [Co(NH3)6]Cl3 was formulated as CoCl3•6NH3. It had mysterious properties, in that it dissolved in water like an ionic solid, but it retained its six ammonia molecules when recrystallized. Even more intriguing was the observation that chemically different forms (isomers) of transition metal complexes such as [Co(NH3)4Cl2]Cl could be made. The puzzle was solved by Alfred Werner, who proposed in 1893 that these Co complexes contained octahedrally coordinated metal ions that made primary (covalent) bonds to six ligands. Werner showed through conductivity measurements that solutions of CoCl3•6NH3 contained three free Cl- anions and one [Co(NH3)6]3+ cation per formula unit. Magnetic susceptibility measurements later confirmed the presence of diamagnetic Co3+ in both the salt and its solutions. Werner's theory also explained the existence of two (and only two) structural isomers for [Co(NH3)4Cl2]+.
|
cis-[Co(NH3)4 Cl2]+ |
|
trans-[Co(NH3)4 Cl2]+ |
Like organic compounds, transition metal complexes can vary widely in size, shape, charge and stability. We will see that bonds formed from the d-orbitals of the metal largely control these properties.
|
Alfred Werner was a Swiss chemist who received the Nobel prize in 1913 for elucidating the bonding in coordination compounds. |