27.1: Organic Reactions: An Introduction
- Page ID
- 24387
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)These are the four "prototypical" organic chemistry reactions, though several others which can be categorized as one of these are generally referred to by other names. Look at these reactions and ask yourself this question for each: what bonds are broken, and what bonds are formed.
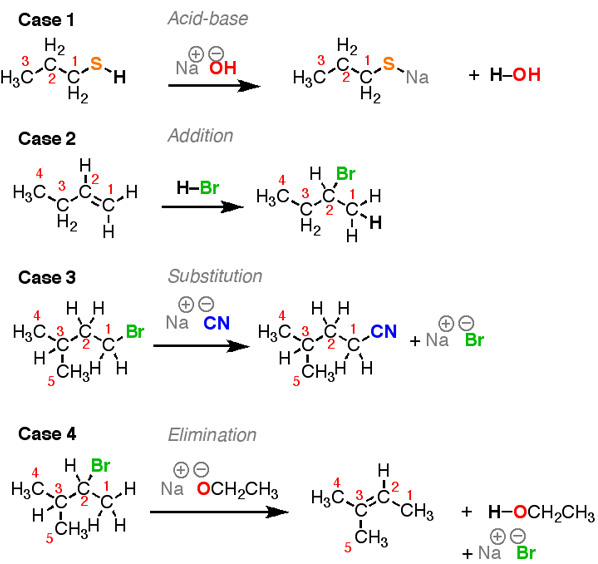
At least 80% of the reactions students in organic chemistry fall into one of these four categories. The sooner you can get into the habit of recognizing bond formation and breakage the better off you will be. A fifth reaction is also discussed: rearrangement reactions.
Acid/Base Reactions
Another important category of organic reactions are straight-forward Brønsted–Lowry acid-base reactions (Figure \(\PageIndex{2}\).
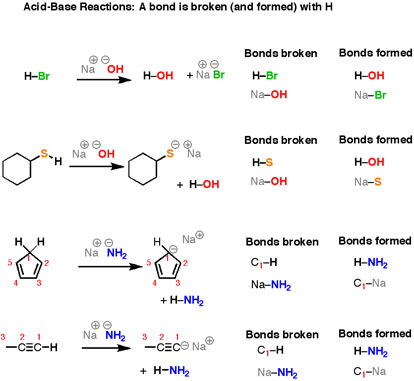
You’ll notice that they might look very different on the surface – all those different structures! – but the basic plot of each reaction is the same. we are breaking an H-(atom) bond and forming an H-(atom) bond. At the same time we are also connecting the two “leftover” partners to form a salt, composed of two oppositely-charged ions. Let’s look at that last reaction in more detail. Here, we are breaking a C-H bond and an (ionic) Na-NH2 bond, and forming an N-H bond as well as an (ionic) C-Na bond.
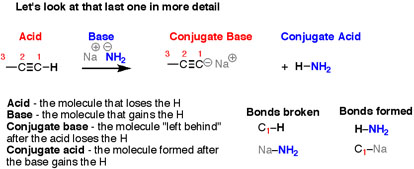
There are four “actors” in this reaction – as there are in every acid-base reaction – and we have names for all of them.
- The reactant where the bond to H is breaking is the acid.
- The reactant where the bond to H is forming is the base
- The product formed when the bond to H is broken is called the conjugate base.
- The product formed when the bond to H is formed is called the conjugate acid.
We can also draw the reverse of the previous reaction. Look at this carefully. we are still breaking a bond to H and forming a bond to H, but we’ve swapped everything. we are breaking N-H and C-Na, and forming N-Na and C-H. It’s still an acid-base reaction.

However, experiment tells us that this reaction does not happen to any appreciable extent.
In this acid/base reaction, which bonds are broken and with are formed, if any?
\[\ce{(CH3-CH2)3N} + \ce{HCl} \rightarrow \ce{(CH3-CH2)3NH^+} + \ce{Cl^-} \nonumber\]
Addition Reactions
When you take an alkene (or alkyne) and add certain types of reagents to them, you get results like this. See if you can recognize the bonds formed and broken.
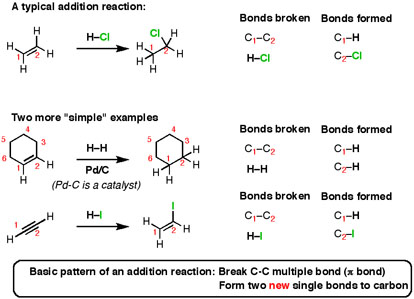
Here’s the basic pattern: break a C-C multiple bond (also called a π bond) and form two new single bonds (“σ-bonds” to carbon). This reaction is called an “addition reaction” and it’s an extremely common type of reaction. Note that the reaction occurs only at the carbons that are a part of a multiple bond – nothing else on the molecule is affected.
In this addition reaction, which bonds are broken and with are formed, if any?
\[\ce{CH3-C(=O)-CH3} \ce{->[\ce{ LiAlH4 }][\ce{ H^+/H2O }]} \ce{CH3-CH(OH)-CH3} \nonumber\]
Note: This reaction could be described as a (nucleophilic) addition reaction or a reduction.
Substitution Reaction
Here are three examples of nucleophilic substitution reactions.
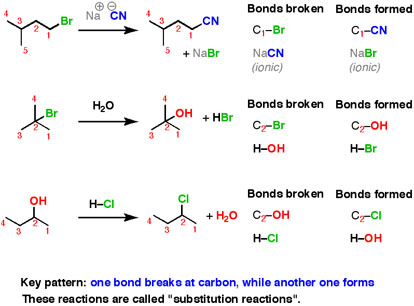
In each case, we are breaking a bond at carbon, and forming a new bond at carbon. This is an extremely common pattern for organic chemistry reactions. Here’s some interesting results that experiments tell us. We don’t get this information by thinking about what happens and predicting – we have to interrogate nature in order to get her to give up her secrets.
In this substitution reaction , which bonds are broken and with are formed, if any?
\[\ce{CH3-C(=O)-OH + CH3-OH} \ce{->[\ce{H2SO4}][\text{ Δ and reflux }]} \ce{CH3-C(=O)-O-CH3} + \ce{H2O} \nonumber\]
Note: This reaction would usually be called a condensation reaction.
Elimination Reactions
Let’s look at two simple cases. One important thing to keep in mind: although you see “Na OCH3” written here, the “Na+” (sodium) is not important for our purposes. It could alternatively be K+ (potassium) or Li+ (lithium). It’s just balancing the negative charge on the oxygen. When you take an alkyl halide and add a strong base (such as \(\ce{NaOCH3}\) or \(\ce{NaOCH2CH3}\)) a reaction occurs. See if you can recognize the bonds broken and formed.
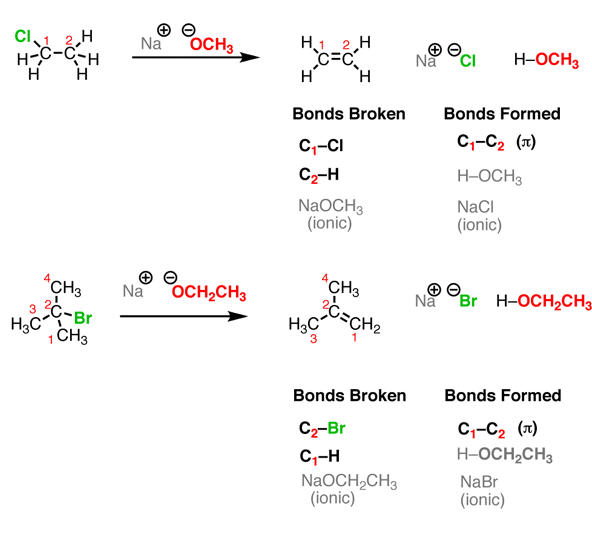
This reaction results in the forming of a new C-C double bond (π bond) and breaking two single bonds to carbon (in these cases, one of them is H and the other is a halide such as Cl or Br). We are also forming a new bond between H and the O. So in this respect, this reaction incorporates a pattern we have seen before – an acid-base reaction. Finally, we also form a salt in this reaction. Since we are primarily interested in the organic product (that is, the one containing carbon), you might find that the salt byproduct is not written in some reaction schemes, but that doesn’t mean that it’s not there.
The three events in the elimination reactyio of Figure \(\PageIndex{3}\) (formation of C-C π bond, breakage of two adjacent carbon sigma bonds) are the exact opposite of the addition reactions discussed above.
Another example of an elimination is when 2-bromopropane is heated under reflux with a concentrated solution of sodium or potassium hydroxide in ethanol. Heating under reflux involves heating with a condenser placed vertically in the flask to avoid loss of volatile liquids. Propene is formed and, because this is a gas, it passes through the condenser and can be collected.
Everything else present (including anything formed in the alternative substitution reaction) will be trapped in the flask.
In this elimination reaction, which bonds are broken and with are formed, if any?
\[\ce{CH3-CH(OH)-CH3} \ce{->[H_2SO_4][K_2Cr_2O_7 ]} \ce{CH3-C(=O)-CH3} \nonumber\]
Note: This reaction could also be described an oxidation reaction.
Rearrangement Reactions
Rearrangement reactions can accompany many of the reactions we’ve previously covered such as substitution, addition, and elimination reactions. These reactions are comparatively rare. In fact, if you don’t look closely, sometimes you can miss the fact that a rearrangement reaction has occurred. Let’s look at a substitution reaction first.
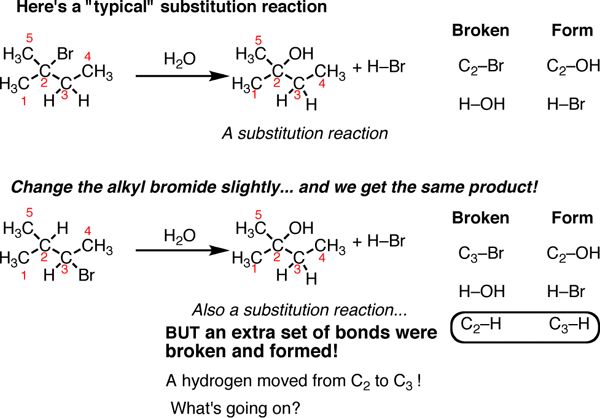
On the top is a “typical” substitution reaction: we are taking an alkyl halide and adding water. The C-Br bond is broken and a C-OH bond is formed. If you look at the table on the right you’ll see this follows the typical pattern of substitution reactions. However if we change one thing about this alkyl halide – move the bromine to C-3 instead of C-2 – now when we run this reaction we see a different product emerge. It is also a substitution reaction (we are replacing Br with OH) but it’s on a different carbon. That’s because if you look closely, you can see there are actually 3 bonds broken and 3 bonds formed. The C2-H bond broke and the C3-H bond formed.
In this Rearrangement reaction, which bonds are broken and with are formed, if any?
\[ \ce{CH3-CH2-CH2-C(OH)=CH2} → \ce{CH3-CH2-CH2-C(=O)-CH3} \nonumber\]
Note: This reaction could also be described an oxidation reaction.
Contributors and Attributions
James Ashenhurst (MasterOrganicChemistry.com)

