24.S: Organic and Biological Chemistry (Summary)
- Page ID
- 70559
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- organic chemistry – the study of carbon compounds
- biochemistry – the study of the chemistry of living species
25.2: Introduction to Hydrocarbons
- made of only hydrogen and carbon
- 4 types: alkanes, alkenes, alkynes, and aromatic hydrocarbons
- alkanes – only single bonds
- also called saturated hydrocarbons
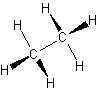
- have the largest amount of hydrogen atoms bonded to a single carbon atom
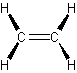
- alkenes (olefins) – have double carbon bonds
-
- alkynes – triple carbon bonds
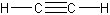
-
- aromatic hydrocarbons – carbon atoms connected in a planar ring structure, joined by s and p bonds between carbon atoms
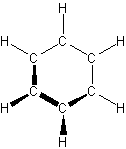
-
- alkenes, alkynes and aromatic hydrocarbons – unsaturated hydrocarbons
- less volatile with increasing molar mass
- very low molecular weight = gas at room temperature
- moderate molecular weight = liquid
- high molecular weight = solid
25.3: Alkanes
- methane major part of natural gas
- used in home heating, gas stoves, hot-water heaters
- propane major part of bottled gas
- used for home heating, cooking
- butane – used in disposable lighters, fuel canisters
- alkanes with 5-12 carbon atoms are found in gasoline
- formla for alkanes is called condensed structural formulas
| Molecular Formula | Condensed Structural Formula | Name | Boiling point (°C) |
|---|---|---|---|
| CH4 | CH4 | Methane | -161 |
| C2H6 | CH3 CH3 | Ethane | -89 |
| C3H8 | CH3 CH2 CH3 | Propane | -44 |
| C4H10 | CH3 CH2 CH2 CH3 | Butane | -0.5 |
| C5H12 | CH3 CH2 CH2 CH2 CH3 | Pentane | 36 |
| C6H14 | CH3 CH2 CH2 CH2 CH2 CH3 | Hexane | 68 |
| C7H16 | CH3 CH2 CH2 CH2 CH2 CH2 CH3 | Heptane | 98 |
| C8H18 | CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH3 | Octane | 125 |
| C9H20 | CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 | Nonane | 151 |
| C10H22 | CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 | Decane | 174 |
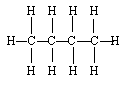
Lewis Structure
- Structures of Alkanes
-
- tetrahedral geometry
- carbon-carbon single bond rotates easily at room temperature
- long chains tend to change shape
- Structural Isomers
-
- straight-chained hydrocarbons – carbons atoms that are joined in a continuous chain
- branched-chain hydrocarbons – hydrocarbons with branched chains, 4 or more carbon atoms
- structural isomers – compounds with the same molecular formula but different bonding arrangements
- Nomenclature of Alkanes
-
- 1) Find the longest continuous chain of carbon atoms, and use the name of this chain as the base name of the compound.
- 2) Number the carbon atoms in the longest chain, beginning with the end of the chain that is nearest to a substituent
- 3) Name and give the location of each substituent group
- 4) When two or more substituents are present list them in alphabetical order
- Cycloalkanes
-
- alkanes that form rings or cycles
- carbon rings with fewer than five carbon atoms are strained
- more reactive
- Reactions of Alkanes
-
- at room temperature alkanes do not react with acids, bases, or strong oxidizing agents
- important for it’s combustion in air; bases for fuels
25.4: Unsaturated Hydrocarbons
- Alkenes
-
- double carbon bonds
- ethene or ethylene simplest alkene\
- nomenclature of alkenes come from the name of the corresponding alkane
- the –ane ending is changed to –ene
- geometrical isomers – compounds that have the same molecular formula and the same groups bonded to one another but differ in the spatial arrangement of these groups
- geometrical isomers have distinct physical properties and differ in chemical behavior
- the double carbon bond is resistant to twisting
- rotation about a double bond is a key process in the chemistry of vision
- Alkynes
-
- one or more triple bonds
- simplest alkyne is acetylene
- highly reactive
- named by changing the alkane ending (-ane) to –yne
- Addition Reactions of Alkenes and Alkynes
-
- addition reactions – a reactant is added to the two atoms that form the multiple bond
- hyrogenation – reaction between al alkene and H2
- Aromatic Hydrocarbons
-
- simplest is benzene
- stability comes from the stabilization of the p electrons through delocalization in the p orbitals
- represented by a hexagon with an inscribed circle
- substitution reactions – one atom of a molecule is removed and replaced by another atom or group of atoms
25.5: Functional Groups
functional group – site of reactivity in an organic molecule; controls how the molecule behaves or functions
- chemistry of an organic molecule is determined by the functional groups it contains
- Alcohols (R-OH)
- alcohols – hydrocarbon derivatives in which one ore more hydrogens of a parent hydrocarbon have been replaced by a hydroxyle or alcohol functional group
- Ethers (R-O-R’)
-
- ethers – two hyrocarbon groups are bonded to one oxygen
- formed from two molecules of alcohol by splitting out a molecule of water
- condensation reaction – reaction where water is split out from two substances
- used in solvents
25.6: Compounds with a Carbonyl Group
-
- carbon oxygen double bonds
- Aldehydes and Ketones
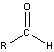
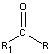
-
- carbonyl group in aldehydes has at least one attached hydrogen atom

formaldehyde
-
- carbonyl group in ketones occurs at the interior of a carbon chain
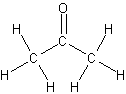
Acetone
-
- prepared by oxidizing alcohols
- Carboxylic Acids
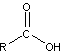
-
- carboxylic acids contain carboxyl functional group (COOH)
- important for manufacturing of polymers
- produced by oxidation in which the OH group is attached to the CH2
- Esters
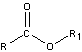
-
- carboxylic acids that undergo condensation with alcohols
- have pleasant odors
- saponification – hydrolysis of an ester in a base
- Amines and Amides
-
- amines – organic bases; general formula R3N
- amides – anines containing a hydrogen bonded to nitrogen that undergoes condensation
25.8: Introduction to Biochemistry
- biosphere – part of the earth where living organisms are formed and living
- includes influences on life of the atmosphere, natural waters, solid earth
- living organisms require a large amount of energy
- biopolymers – three categories: proteins, polysaccharides (carbohydrates), and nucleic acids
25.9: Proteins
- macromolecular substances
- 50% body’s dry weight is proteins
- composed of amino acids
- Amino Acids
- differ in the R group
- building block of all proteins - a -amino acid
- general form:

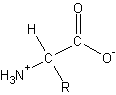
-
- chiral - any molecule containing a carbon with four different attached groups
- enantiomers – mirror-image of chiral
- enantiomers and chiral have the same physical properties
- differ in chemical reactivity toward other chiral molecules
- Polypeptides and Proteins
- peptide bond – condesation reaction between the carboxyl group of one amino acid and the amino group of another amino acid
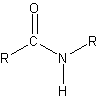
- polypeptides – large number of amino acids that are linked together by peptide bonds
- proteins are polypeptide molecules
- weighs from 6000 to over 50 million amu
- Protein Structure
-
- primary structure – arrangement of amino acids along a protein chain
- makes the protein unique
- secondary structure – the way segments of the protein chain are oriented in a regular pattern
- a -helix – most important and common secondary structure arrangement
- first propoesed by Linus Pauling and R. B. Corey
- tertiary structure – overal shape of a protein
- globular protein – a protein that folds into a compact spherical shape
- soluble in water, mobile within cells
- enzymes – large protein molecules that serve as catalysts
25.10: Carbohydrates
-
- written as Cx(H2O)y
- glucose the most abundant carbohydrate C6H12O6
- not really hydrates of carbon but polyhydroxly aldehydes and ketones
- Disaccharides
-
- monosaccharides – simple sugars that can’t be broken into smaller molecules by hydrolysis with aqueous acids
- disaccharide – two linked monosaccharides
- two common disaccharides: sucrose, lactose
- Polysaccharides
-
- made of several monosaccharide units
- starch – group of polysaccharides
- food storage in plant seeds and tuers
- glycogen – starchlike substance synthesized in the body
- 5000 to more than 5 million amu
- energy bank in the body; muscles and liver
- cellulose – major strructural unit of plants
- straight chains of glucose units
- more than 500,000 amu
25.11: Nucleic Acids
- nucleic acids – biopolymers that are chemical carriers of an organisn’t genetic information
- deoxyribonucleic acids (DNA) – huge molecules with molar weights of 6-16million amu
- ribonucleic acids (RNA) – smaller molecules with molecular weights of 20,000 to 40,000 amu
- DNA found inside the nucleus of a cell, RNA found outside nucleus in the cytoplasm
- DNA stores the genetic information of the cell and controls the production of proteins
- RNA carries information from the DNA out of the nucleus
- Monomers of nucleic acids (nucleotides) have three parts:
- A phosphoric acid molecule, H3PO4
- A five-carbon sugar
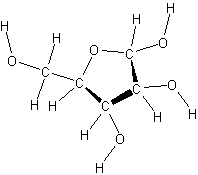
ribose
- A nitrogen-containing organic base
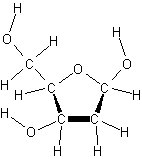
deoxyribose
-
- deoxyribose has one less oxygen atom at carbon 2 than ribose
- nucleic acids are polynucleotides
- DNA molecules made of two DNA chains that form a double helix

