17.7: Qualitative Analysis for Metallic Elements
- Page ID
- 25189
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To know how to separate metal ions by selective precipitation.
- To understand how several common metal cations can be identified in a solution using selective precipitation.
The composition of relatively complex mixtures of metal ions can be determined using qualitative analysis, a procedure for discovering the identity of metal ions present in the mixture (rather than quantitative information about their amounts). The procedure used to separate and identify more than 20 common metal cations from a single solution consists of selectively precipitating only a few kinds of metal ions at a time under given sets of conditions. Consecutive precipitation steps become progressively less selective until almost all of the metal ions are precipitated, as illustrated in Figure \(\PageIndex{1}\).
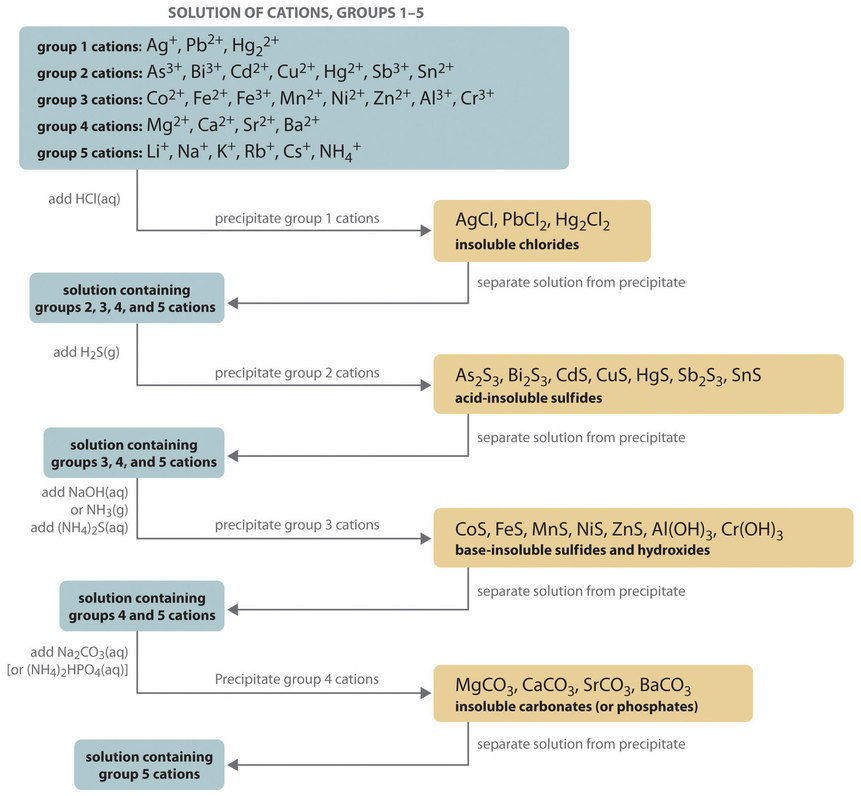
Group 1: Insoluble Chlorides
Most metal chloride salts are soluble in water; only \(\ce{Ag^{+}}\), \(\ce{Pb^{2+}}\), and \(\ce{Hg2^{2+}}\) form chlorides that precipitate from water. Thus the first step in a qualitative analysis is to add about 6 M \(\ce{HCl}\), thereby causing \(\ce{AgCl}\), \(\ce{PbCl2}\), and/or \(\ce{Hg2Cl2}\) to precipitate. If no precipitate forms, then these cations are not present in significant amounts. The precipitate can be collected by filtration or centrifugation.
Group 2: Acid-Insoluble Sulfides
Next, the acidic solution is saturated with \(\ce{H2S}\) gas. Only those metal ions that form very insoluble sulfides, such as \(\ce{As^{3+}}\), \(\ce{Bi^{3+}}\), \(\ce{Cd^{2+}}\), \(\ce{Cu^{2+}}\), \(\ce{Hg^{2+}}\), \(\ce{Sb^{3+}}\), and \(\ce{Sn^{2+}}\), precipitate as their sulfide salts under these acidic conditions. All others, such as \(\ce{Fe^{2+}}\) and \(\ce{Zn^{2+}}\), remain in solution. Once again, the precipitates are collected by filtration or centrifugation.
Group 3: Base-Insoluble Sulfides (and Hydroxides)
Ammonia or \(\ce{NaOH}\) is now added to the solution until it is basic, and then \(\ce{(NH4)2S}\) is added. This treatment removes any remaining cations that form insoluble hydroxides or sulfides. The divalent metal ions \(\ce{Co^{2+}}\), \(\ce{Fe^{2+}}\), \(\ce{Mn^{2+}}\), \(\ce{Ni^{2+}}\), and \(\ce{Zn^{2+}}\) precipitate as their sulfides, and the trivalent metal ions \(\ce{Al^{3+}}\) and \(\ce{Cr^{3+}}\) precipitate as their hydroxides: \(\ce{Al(OH)3}\) and \(\ce{Cr(OH)3}\). If the mixture contains \(\ce{Fe^{3+}}\), sulfide reduces the cation to \(\ce{Fe^{2+}}\), which precipitates as \(\ce{FeS}\).
Group 4: Insoluble Carbonates or Phosphates
The next metal ions to be removed from solution are those that form insoluble carbonates and phosphates. When \(\ce{Na2CO3}\) is added to the basic solution that remains after the precipitated metal ions are removed, insoluble carbonates precipitate and are collected. Alternatively, adding \(\ce{(NH4)2HPO4}\) causes the same metal ions to precipitate as insoluble phosphates.
Group 5: Alkali Metals
At this point, we have removed all the metal ions that form water-insoluble chlorides, sulfides, carbonates, or phosphates. The only common ions that might remain are any alkali metals (\(\ce{Li^{+}}\), \(\ce{Na^{+}}\), \(\ce{K^{+}}\), \(\ce{Rb^{+}}\), and \(\ce{Cs^{+}}\)) and ammonium (\(\ce{NH4^{+}}\)). We now take a second sample from the original solution and add a small amount of \(\ce{NaOH}\) to neutralize the ammonium ion and produce \(\ce{NH3}\). (We cannot use the same sample we used for the first four groups because we added ammonium to that sample in earlier steps.) Any ammonia produced can be detected by either its odor or a litmus paper test. A flame test on another original sample is used to detect sodium, which produces a characteristic bright yellow color. The other alkali metal ions also give characteristic colors in flame tests, which allows them to be identified if only one is present.
Metal ions that precipitate together are separated by various additional techniques, such as forming complex ions, changing the pH of the solution, or increasing the temperature to redissolve some of the solids. For example, the precipitated metal chlorides of group 1 cations, containing \(\ce{Ag^{+}}\), \(\ce{Pb^{2+}}\), and \(\ce{Hg2^{2+}}\), are all quite insoluble in water. Because \(\ce{PbCl2}\) is much more soluble in hot water than are the other two chloride salts, however, adding water to the precipitate and heating the resulting slurry will dissolve any \(\ce{PbCl2}\) present. Isolating the solution and adding a small amount of \(\ce{Na2CrO4}\) solution to it will produce a bright yellow precipitate of \(\ce{PbCrO4}\) if \(\ce{Pb^{2+}}\) were in the original sample (Figure \(\PageIndex{2}\)).
As another example, treating the precipitates from group 1 cations with aqueous ammonia will dissolve any \(\ce{AgCl}\) because \(\ce{Ag^{+}}\) forms a stable complex with ammonia: \(\ce{[Ag(NH3)2]^{+}}\). In addition, \(\ce{Hg2Cl2}\) disproportionates in ammonia.
\[\ce{2Hg2^{2+} \rightarrow Hg + Hg^{2+}} \nonumber \]
to form a black solid that is a mixture of finely divided metallic mercury and an insoluble mercury(II) compound, which is separated from solution:
\[\ce{Hg2Cl2(s) + 2NH3(aq) \rightarrow Hg(l) + Hg(NH_2)Cl(s) + NH^{+}4(aq) + Cl^{−}(aq)} \nonumber \]
Any silver ion in the solution is then detected by adding \(\ce{HCl}\), which reverses the reaction and gives a precipitate of white \(\ce{AgCl}\) that slowly darkens when exposed to light:
\[\ce{[Ag(NH3)2]^{+} (aq) + 2H^{+}(aq) + Cl^{−}(aq) \rightarrow AgCl(s) + 2NH^{+}4(aq)} \nonumber \]
Similar but slightly more complex reactions are also used to separate and identify the individual components of the other groups.
Summary
In qualitative analysis, the identity, not the amount, of metal ions present in a mixture is determined. The technique consists of selectively precipitating only a few kinds of metal ions at a time under given sets of conditions. Consecutive precipitation steps become progressively less selective until almost all the metal ions are precipitated. Other additional steps are needed to separate metal ions that precipitate together.


