2.8: Naming Inorganic Compounds
- Page ID
- 21704
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)↵
- To describe the composition of a chemical compound.
- To name covalent compounds that contain up to three elements.
As with ionic compounds, the system for naming covalent compounds enables chemists to write the molecular formula from the name and vice versa. This and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic compounds that contain only carbon and hydrogen.
When chemists synthesize a new compound, they may not yet know its molecular or structural formula. In such cases, they usually begin by determining its empirical formula, the relative numbers of atoms of the elements in a compound, reduced to the smallest whole numbers. Because the empirical formula is based on experimental measurements of the numbers of atoms in a sample of the compound, it shows only the ratios of the numbers of the elements present. The difference between empirical and molecular formulas can be illustrated with butane, a covalent compound used as the fuel in disposable lighters. The molecular formula for butane is \(\ce{C4H10}\). The ratio of carbon atoms to hydrogen atoms in butane is 4:10, which can be reduced to 2:5. The empirical formula for butane is therefore \(\ce{C2H5}\). The formula unit is the absolute grouping of atoms or ions represented by the empirical formula of a compound, either ionic or covalent. Butane has the empirical formula \(\ce{C2H5}\), but it contains two \(\ce{C2H5}\) formula units, giving a molecular formula of \(\ce{C4H10}\).
Because ionic compounds do not contain discrete molecules, empirical formulas are used to indicate their compositions. All compounds, whether ionic or covalent, must be electrically neutral. Consequently, the positive and negative charges in a formula unit must exactly cancel each other. If the cation and the anion have charges of equal magnitude, such as \(\ce{Na^{+}}\) and \(\ce{Cl^{−}}\), then the compound must have a 1:1 ratio of cations to anions, and the empirical formula must be \(\ce{NaCl}\). If the charges are not the same magnitude, then a cation:anion ratio other than 1:1 is needed to produce a neutral compound. In the case of \(\ce{Mg^{2+}}\) and \(\ce{Cl^{−}}\), for example, two Cl− ions are needed to balance the two positive charges on each Mg2+ ion, giving an empirical formula of \(\ce{MgCl2}\). Similarly, the formula for the ionic compound that contains Na+ and O2− ions is Na2O.
Ionic compounds do not contain discrete molecules, so empirical formulas are used to indicate their compositions.
Binary Ionic Compounds
An ionic compound that contains only two elements, one present as a cation and one as an anion, is called a binary ionic compound. One example is \(\ce{MgCl_2}\), a coagulant used in the preparation of tofu from soybeans. For binary ionic compounds, the subscripts in the empirical formula can also be obtained by crossing charges: use the absolute value of the charge on one ion as the subscript for the other ion. This method is shown schematically as follows:
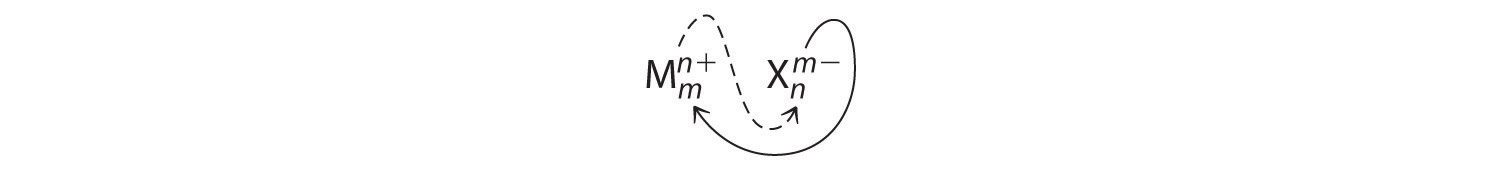
When crossing charges, it is sometimes necessary to reduce the subscripts to their simplest ratio to write the empirical formula. Consider, for example, the compound formed by Mg2+ and O2−. Using the absolute values of the charges on the ions as subscripts gives the formula \(\ce{Mg2O2}\):
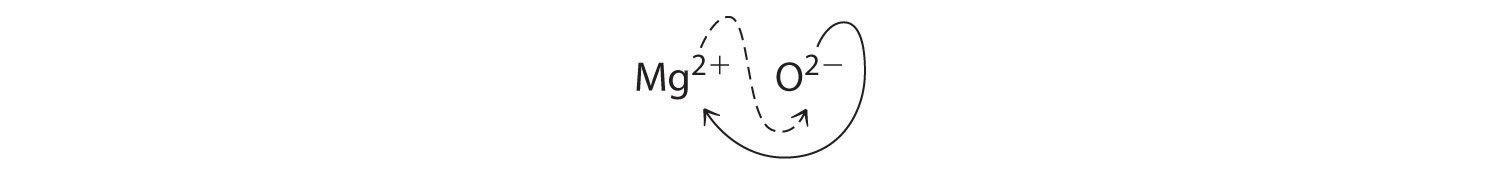
This simplifies to its correct empirical formula MgO. The empirical formula has one Mg2+ ion and one O2− ion.
Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair.
- Ga3+ and As3−
- Eu3+ and O2−
- calcium and chlorine
Given: ions or elements
Asked for: empirical formula for binary ionic compound
Strategy:
- If not given, determine the ionic charges based on the location of the elements in the periodic table.
- Use the absolute value of the charge on each ion as the subscript for the other ion. Reduce the subscripts to the lowest numbers
to write the empirical formula. Check to make sure the empirical formula is electrically neutral.
Solution
a. B Using the absolute values of the charges on the ions as the subscripts gives \(\ce{Ga3As3}\):
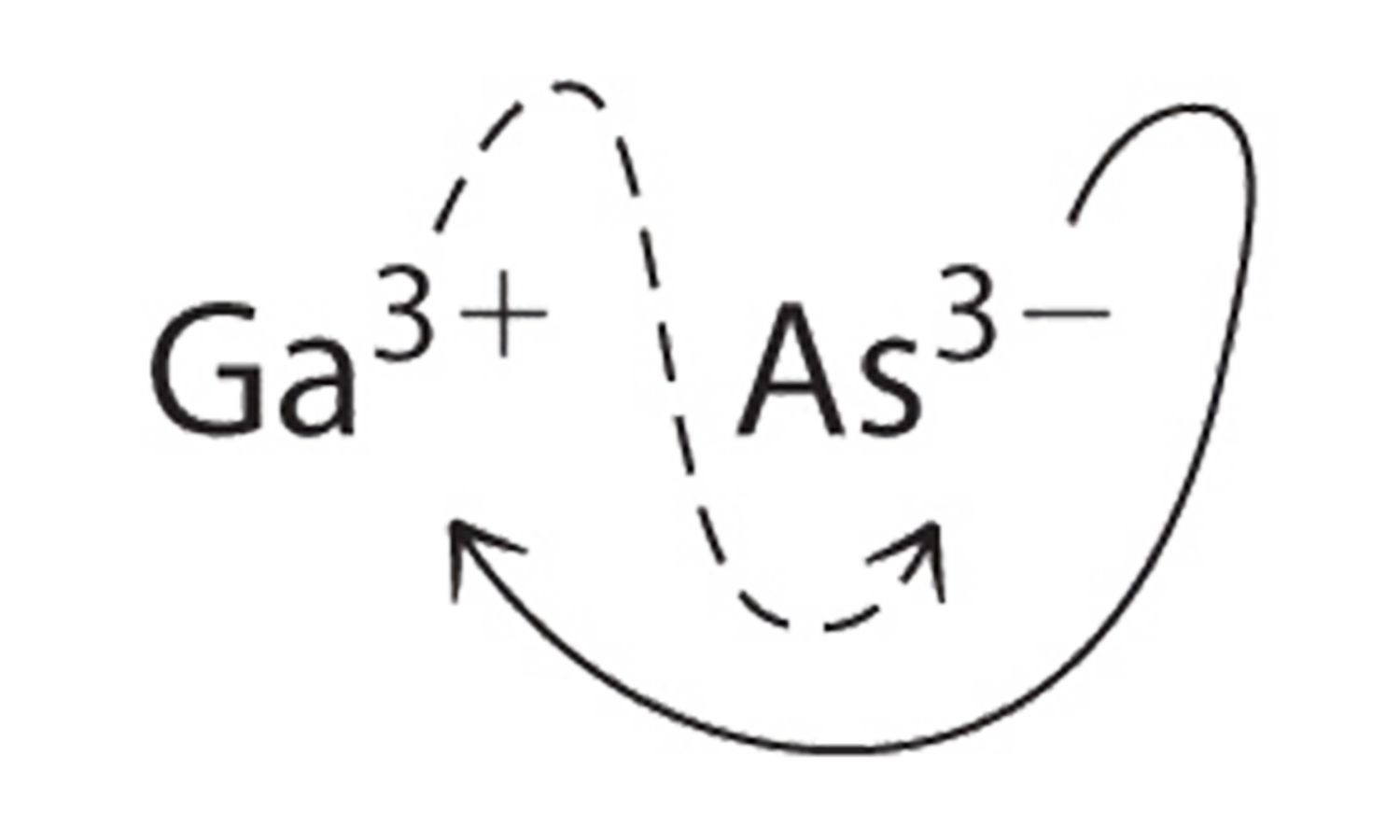
Reducing the subscripts to the smallest whole numbers gives the empirical formula GaAs, which is electrically neutral [+3 + (−3) = 0]. Alternatively, we could recognize that Ga3+ and As3− have charges of equal magnitude but opposite signs. One Ga3+ ion balances the charge on one As3− ion, and a 1:1 compound will have no net charge. Because we write subscripts only if the number is greater than 1, the empirical formula is GaAs. GaAs is gallium arsenide, which is widely used in the electronics industry in transistors and other devices.
b. B Because Eu3+ has a charge of +3 and O2− has a charge of −2, a 1:1 compound would have a net charge of +1. We must therefore find multiples of the charges that cancel. We cross charges, using the absolute value of the charge on one ion as the subscript for the other ion:
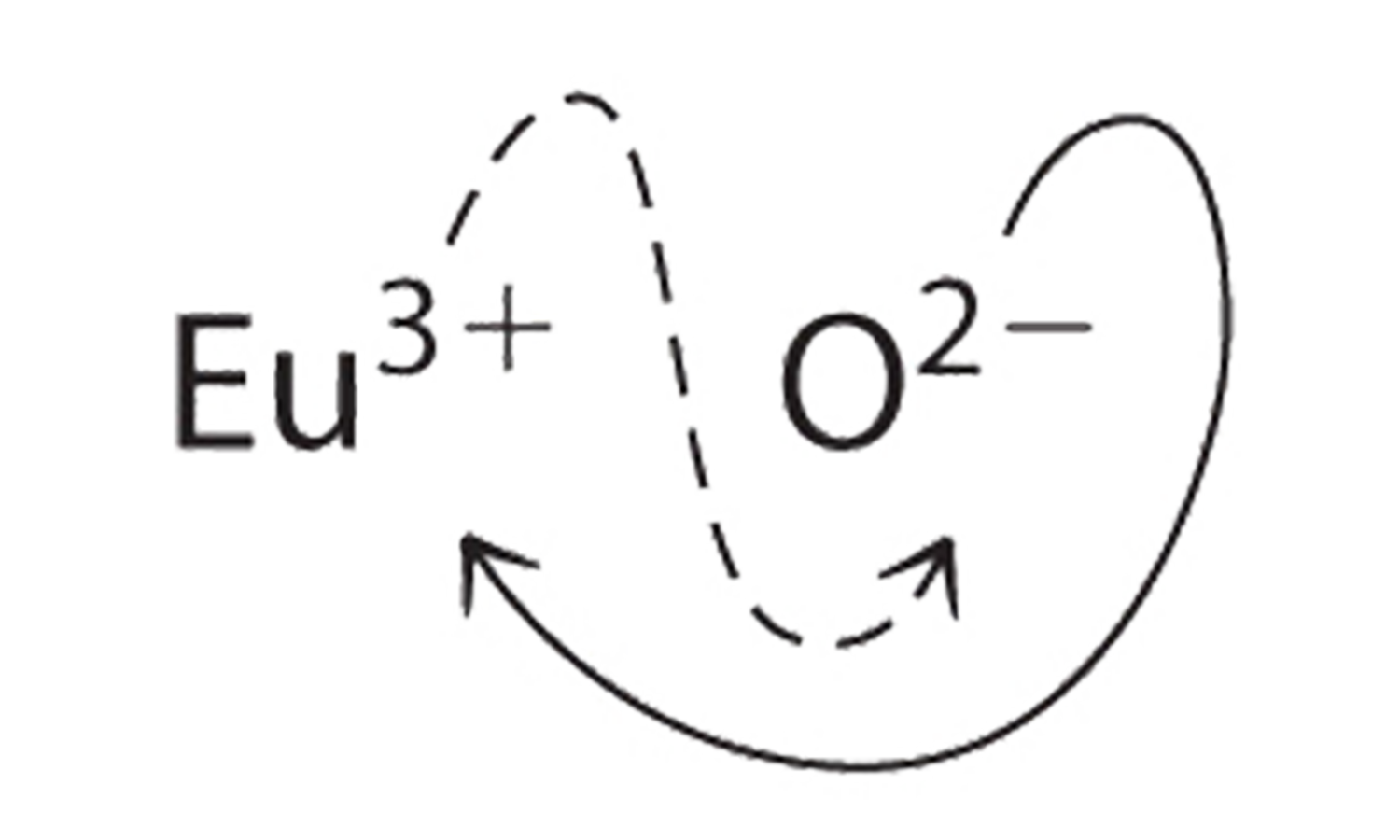
The subscript for Eu3+ is 2 (from O2−), and the subscript for O2− is 3 (from Eu3+), giving Eu2O3; the subscripts cannot be reduced further. The empirical formula contains a positive charge of 2(+3) = +6 and a negative charge of 3(−2) = −6, for a net charge of 0. The compound Eu2O3 is neutral. Europium oxide is responsible for the red color in television and computer screens.
c. A Because the charges on the ions are not given, we must first determine the charges expected for the most common ions derived from calcium and chlorine. Calcium lies in group 2, so it should lose two electrons to form Ca2+. Chlorine lies in group 17, so it should gain one electron to form Cl−.
B Two Cl− ions are needed to balance the charge on one Ca2+ ion, which leads to the empirical formula CaCl2. We could also cross charges, using the absolute value of the charge on Ca2+ as the subscript for Cl and the absolute value of the charge on Cl− as the subscript for Ca:

The subscripts in CaCl2 cannot be reduced further. The empirical formula is electrically neutral [+2 + 2(−1) = 0]. This compound is calcium chloride, one of the substances used as “salt” to melt ice on roads and sidewalks in winter.
Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair.
- Li+ and N3−
- Al3+ and O2−
- lithium and oxygen
- Answer a
-
Li3N
- Answer b
-
Al2O3
- Answer c
-
Li2O
Nomenclature of Metals: Nomenclature of Metals(opens in new window) [youtu.be]
Polyatomic Ions
Polyatomic ions are groups of atoms that bear net electrical charges, although the atoms in a polyatomic ion are held together by the same covalent bonds that hold atoms together in molecules. Just as there are many more kinds of molecules than simple elements, there are many more kinds of polyatomic ions than monatomic ions. Two examples of polyatomic cations are the ammonium (NH4+) and the methylammonium (CH3NH3+) ions. Polyatomic anions are much more numerous than polyatomic cations; some common examples are in Table \(\PageIndex{1}\).
| Formula | Name of Ion | Formula | Name of Ion |
|---|---|---|---|
| NH4+ | ammonium | HPO42− | hydrogen phosphate |
| CH3NH3+ | methylammonium | H2PO4− | dihydrogen phosphate |
| OH− | hydroxide | ClO− | hypochlorite |
| O22− | peroxide | ClO2− | chlorite |
| CN− | cyanide | ClO3− | chlorate |
| SCN− | thiocyanate | ClO4− | perchlorate |
| NO2− | nitrite | MnO4− | permanganate |
| NO3− | nitrate | CrO42− | chromate |
| CO32− | carbonate | Cr2O72− | dichromate |
| HCO3− | hydrogen carbonate, or bicarbonate | C2O42− | oxalate |
| SO32− | sulfite | HCO2− | formate |
| SO42− | sulfate | CH3CO2− | acetate |
| HSO4− | hydrogen sulfate, or bisulfate | C6H5CO2− | benzoate |
| PO43− | phosphate |
The method used to predict the empirical formulas for ionic compounds that contain monatomic ions can also be used for compounds that contain polyatomic ions. The overall charge on the cations must balance the overall charge on the anions in the formula unit. Thus, K+ and NO3− ions combine in a 1:1 ratio to form KNO3 (potassium nitrate or saltpeter), a major ingredient in black gunpowder. Similarly, Ca2+ and SO42− form CaSO4 (calcium sulfate), which combines with varying amounts of water to form gypsum and plaster of Paris. The polyatomic ions NH4+ and NO3− form NH4NO3 (ammonium nitrate), a widely used fertilizer and, in the wrong hands, an explosive. One example of a compound in which the ions have charges of different magnitudes is calcium phosphate, which is composed of Ca2+ and PO43− ions; it is a major component of bones. The compound is electrically neutral because the ions combine in a ratio of three Ca2+ ions [3(+2) = +6] for every two ions [2(−3) = −6], giving an empirical formula of Ca3(PO4)2; the parentheses around PO4 in the empirical formula indicate that it is a polyatomic ion. Writing the formula for calcium phosphate as Ca3P2O8 gives the correct number of each atom in the formula unit, but it obscures the fact that the compound contains readily identifiable PO43− ions.
Write the empirical formula for the compound formed from each ion pair.
- Na+ and HPO42−
- potassium cation and cyanide anion
- calcium cation and hypochlorite anion
Given: ions
Asked for: empirical formula for ionic compound
Strategy:
- If it is not given, determine the charge on a monatomic ion from its location in the periodic table. Use Table \(\PageIndex{1}\) to find the charge on a polyatomic ion.
- Use the absolute value of the charge on each ion as the subscript for the other ion. Reduce the subscripts to the smallest whole numbers when writing the empirical formula.
Solution:
- B Because HPO42− has a charge of −2 and Na+ has a charge of +1, the empirical formula requires two Na+ ions to balance the charge of the polyatomic ion, giving Na2HPO4. The subscripts are reduced to the lowest numbers, so the empirical formula is Na2HPO4. This compound is sodium hydrogen phosphate, which is used to provide texture in processed cheese, puddings, and instant breakfasts.
- A The potassium cation is K+, and the cyanide anion is CN−. B Because the magnitude of the charge on each ion is the same, the empirical formula is KCN. Potassium cyanide is highly toxic, and at one time it was used as rat poison. This use has been discontinued, however, because too many people were being poisoned accidentally.
- A The calcium cation is Ca2+, and the hypochlorite anion is ClO−. B Two ClO− ions are needed to balance the charge on one Ca2+ ion, giving Ca(ClO)2. The subscripts cannot be reduced further, so the empirical formula is Ca(ClO)2. This is calcium hypochlorite, the “chlorine” used to purify water in swimming pools.
Write the empirical formula for the compound formed from each ion pair.
- Ca2+ and H2PO4−
- sodium cation and bicarbonate anion
- ammonium cation and sulfate anion
- Answer a
-
Ca(H2PO4)2: calcium dihydrogen phosphate is one of the ingredients in baking powder.
- Answer b
-
NaHCO3: sodium bicarbonate is found in antacids and baking powder; in pure form, it is sold as baking soda.
- Answer c
-
(NH4)2SO4: ammonium sulfate is a common source of nitrogen in fertilizers.
Polyatomics: Polyatomics, YouTube(opens in new window) [youtu.be] (opens in new window)
Hydrates
Many ionic compounds occur as hydrates, compounds that contain specific ratios of loosely bound water molecules, called waters of hydration. Waters of hydration can often be removed simply by heating. For example, calcium dihydrogen phosphate can form a solid that contains one molecule of water per \(\ce{Ca(H2PO4)2}\) unit and is used as a leavening agent in the food industry to cause baked goods to rise. The empirical formula for the solid is \(\ce{Ca(H2PO4)2·H2O}\). In contrast, copper sulfate usually forms a blue solid that contains five waters of hydration per formula unit, with the empirical formula \(\ce{CuSO4·5H2O}\). When heated, all five water molecules are lost, giving a white solid with the empirical formula \(\ce{CuSO4}\).


Compounds that differ only in the numbers of waters of hydration can have very different properties. For example, \(\ce{CaSO4·½H2O}\) is plaster of Paris, which was often used to make sturdy casts for broken arms or legs, whereas \(\ce{CaSO4·2H2O}\) is the less dense, flakier gypsum, a mineral used in drywall panels for home construction. When a cast would set, a mixture of plaster of Paris and water crystallized to give solid \(\ce{CaSO4·2H2O}\). Similar processes are used in the setting of cement and concrete.
Binary Acids
Some compounds containing hydrogen are members of an important class of substances known as acids. The chemistry of these compounds is explored in more detail in later chapters of this text, but for now, it will suffice to note that many acids release hydrogen ions, H+, when dissolved in water. To denote this distinct chemical property, a mixture of water with an acid is given a name derived from the compound’s name. If the compound is a binary acid (comprised of hydrogen and one other nonmetallic element):
- The word “hydrogen” is changed to the prefix hydro-
- The other nonmetallic element name is modified by adding the suffix -ic
- The word “acid” is added as a second word
For example, when the gas \(\ce{HCl}\) (hydrogen chloride) is dissolved in water, the solution is called hydrochloric acid. Several other examples of this nomenclature are shown in Table \(\PageIndex{2}\).
| Name of Gas | Name of Acid |
|---|---|
| HF(g), hydrogen fluoride | HF(aq), hydrofluoric acid |
| HCl(g), hydrogen chloride | HCl(aq), hydrochloric acid |
| HBr(g), hydrogen bromide | HBr(aq), hydrobromic acid |
| HI(g), hydrogen iodide | HI(aq), hydroiodic acid |
| H2S(g), hydrogen sulfide | H2S(aq), hydrosulfuric acid |
Oxyacids
Many compounds containing three or more elements (such as organic compounds or coordination compounds) are subject to specialized nomenclature rules that you will learn later. However, we will briefly discuss the important compounds known as oxyacids, compounds that contain hydrogen, oxygen, and at least one other element, and are bonded in such a way as to impart acidic properties to the compound (you will learn the details of this in a later chapter). Typical oxyacids consist of hydrogen combined with a polyatomic, oxygen-containing ion. To name oxyacids:
- Omit “hydrogen”
- Start with the root name of the anion
- Replace –ate with –ic, or –ite with –ous
- Add “acid”
For example, consider H2CO3 (which you might be tempted to call “hydrogen carbonate”). To name this correctly, “hydrogen” is omitted; the –ate of carbonate is replace with –ic; and acid is added—so its name is carbonic acid. Other examples are given in Table \(\PageIndex{3}\). There are some exceptions to the general naming method (e.g., H2SO4 is called sulfuric acid, not sulfic acid, and H2SO3 is sulfurous, not sulfous, acid).
| Formula | Anion Name | Acid Name |
|---|---|---|
| HC2H3O2 | acetate | acetic acid |
| HNO3 | nitrate | nitric acid |
| HNO2 | nitrite | nitrous acid |
| HClO4 | perchlorate | perchloric acid |
| H2CO3 | carbonate | carbonic acid |
| H2SO4 | sulfate | sulfuric acid |
| H2SO3 | sulfite | sulfurous acid |
| H3PO4 | phosphate | phosphoric acid |
Nomenclature of Acids: Nomenclature of Acids, YouTube(opens in new window) [youtu.be]
Bases
We will present more comprehensive definitions of bases in later chapters, but virtually every base you encounter in the meantime will be an ionic compound, such as sodium hydroxide (NaOH) and barium hydroxide [Ba(OH)2], that contain the hydroxide ion and a metal cation. These have the general formula M(OH)n. It is important to recognize that alcohols, with the general formula ROH, are covalent compounds, not ionic compounds; consequently, they do not dissociate in water to form a basic solution (containing OH− ions). When a base reacts with any of the acids we have discussed, it accepts a proton (H+). For example, the hydroxide ion (OH−) accepts a proton to form H2O. Thus bases are also referred to as proton acceptors.
Concentrated aqueous solutions of ammonia (NH3) contain significant amounts of the hydroxide ion, even though the dissolved substance is not primarily ammonium hydroxide (NH4OH) as is often stated on the label. Thus aqueous ammonia solution is also a common base. Replacing a hydrogen atom of NH3 with an alkyl group results in an amine (RNH2), which is also a base. Amines have pungent odors—for example, methylamine (CH3NH2) is one of the compounds responsible for the foul odor associated with spoiled fish. The physiological importance of amines is suggested in the word vitamin, which is derived from the phrase vital amines. The word was coined to describe dietary substances that were effective at preventing scurvy, rickets, and other diseases because these substances were assumed to be amines. Subsequently, some vitamins have indeed been confirmed to be amines.
Binary Inorganic Compounds
Binary covalent compounds—covalent compounds that contain only two elements—are named using a procedure similar to that used for simple ionic compounds, but prefixes are added as needed to indicate the number of atoms of each kind. The procedure, diagrammed in Figure \(\PageIndex{2}\) consists of the following steps:
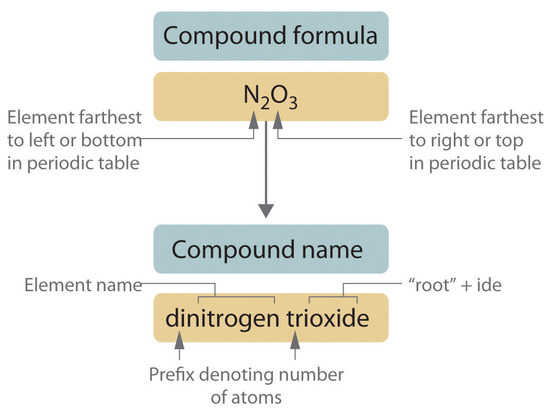
- Place the elements in their proper order.
- The element farthest to the left in the periodic table is usually named first. If both elements are in the same group, the element closer to the bottom of the column is named first.
- The second element is named as if it were a monatomic anion in an ionic compound (even though it is not), with the suffix -ide attached to the root of the element name.
- Identify the number of each type of atom present.
- Prefixes derived from Greek stems are used to indicate the number of each type of atom in the formula unit (Table \(\PageIndex{3}\)). The prefix mono- (“one”) is used only when absolutely necessary to avoid confusion, just as the subscript 1 is omitted when writing molecular formulas.
To demonstrate steps 1 and 2a, HCl is named hydrogen chloride (because hydrogen is to the left of chlorine in the periodic table), and PCl5 is phosphorus pentachloride. The order of the elements in the name of BrF3, bromine trifluoride, is determined by the fact that bromine lies below fluorine in Group 17.
Table \(\PageIndex{3}\): Prefixes for Indicating the Number of Atoms in Chemical Names Prefix Number mono- 1 di- 2 tri- 3 tetra- 4 penta- 5 hexa- 6 hepta- 7 octa- 8 nona- 9 deca- 10 undeca- 11 dodeca- 12 - If a molecule contains more than one atom of both elements, then prefixes are used for both. Thus N2O3 is dinitrogen trioxide, as shown in Figure 2.13.
- In some names, the final a or o of the prefix is dropped to avoid awkward pronunciation. Thus OsO4 is osmium tetroxide rather than osmium tetraoxide.
- Prefixes derived from Greek stems are used to indicate the number of each type of atom in the formula unit (Table \(\PageIndex{3}\)). The prefix mono- (“one”) is used only when absolutely necessary to avoid confusion, just as the subscript 1 is omitted when writing molecular formulas.
- Write the name of the compound.
- Binary compounds of the elements with oxygen are generally named as “element oxide,” with prefixes that indicate the number of atoms of each element per formula unit. For example, CO is carbon monoxide. The only exception is binary compounds of oxygen with fluorine, which are named as oxygen fluorides.
- Certain compounds are always called by the common names that were assigned before formulas were used. For example, H2O is water (not dihydrogen oxide); NH3 is ammonia; PH3 is phosphine; SiH4 is silane; and B2H6, a dimer of BH3, is diborane. For many compounds, the systematic name and the common name are both used frequently, requiring familiarity with both. For example, the systematic name for NO is nitrogen monoxide, but it is much more commonly called nitric oxide. Similarly, N2O is usually called nitrous oxide rather than dinitrogen monoxide. Notice that the suffixes -ic and -ous are the same ones used for ionic compounds.
Start with the element at the far left in the periodic table and work to the right. If two or more elements are in the same group, start with the bottom element and work up.
Write the name of each binary covalent compound.
- SF6
- N2O4
- ClO2
Given: molecular formula
Asked for: name of compound
Strategy:
- List the elements in order according to their positions in the periodic table. Identify the number of each type of atom in the chemical formula and then use Table \(\PageIndex{2}\) to determine the prefixes needed.
- If the compound contains oxygen, follow step 3a. If not, decide whether to use the common name or the systematic name.
Solution:
- A Because sulfur is to the left of fluorine in the periodic table, sulfur is named first. Because there is only one sulfur atom in the formula, no prefix is needed. B There are, however, six fluorine atoms, so we use the prefix for six: hexa- (Table \(\PageIndex{2}\)). The compound is sulfur hexafluoride.
- A Because nitrogen is to the left of oxygen in the periodic table, nitrogen is named first. Because more than one atom of each element is present, prefixes are needed to indicate the number of atoms of each. According to Table \(\PageIndex{2}\) "Prefixes for Indicating the Number of Atoms in Chemical Names", the prefix for two is di-, and the prefix for four is tetra-. B The compound is dinitrogen tetroxide (omitting the a in tetra- according to step 2c) and is used as a component of some rocket fuels.
- A Although oxygen lies to the left of chlorine in the periodic table, it is not named first because ClO2 is an oxide of an element other than fluorine (step 3a). Consequently, chlorine is named first, but a prefix is not necessary because each molecule has only one atom of chlorine. B Because there are two oxygen atoms, the compound is a dioxide. Thus the compound is chlorine dioxide. It is widely used as a substitute for chlorine in municipal water treatment plants because, unlike chlorine, it does not react with organic compounds in water to produce potentially toxic chlorinated compounds.
Write the name of each binary covalent compound.
- IF7
- N2O5
- OF2
- Answer a
-
iodine heptafluoride
- Answer b
-
dinitrogen pentoxide
- Answer c
-
oxygen difluoride
Write the formula for each binary covalent compound.
- sulfur trioxide
- diiodine pentoxide
Given: name of compound
Asked for: formula
Strategy:
List the elements in the same order as in the formula, use Table \(\PageIndex{2}\) to identify the number of each type of atom present, and then indicate this quantity as a subscript to the right of that element when writing the formula.
Solution:
- Sulfur has no prefix, which means that each molecule has only one sulfur atom. The prefix tri- indicates that there are three oxygen atoms. The formula is therefore SO3. Sulfur trioxide is produced industrially in huge amounts as an intermediate in the synthesis of sulfuric acid.
- The prefix di- tells you that each molecule has two iodine atoms, and the prefix penta- indicates that there are five oxygen atoms. The formula is thus I2O5, a compound used to remove carbon monoxide from air in respirators.
Write the formula for each binary covalent compound.
- silicon tetrachloride
- disulfur decafluoride
- Answer a
-
SiCl4
- Answer b
-
S2F10
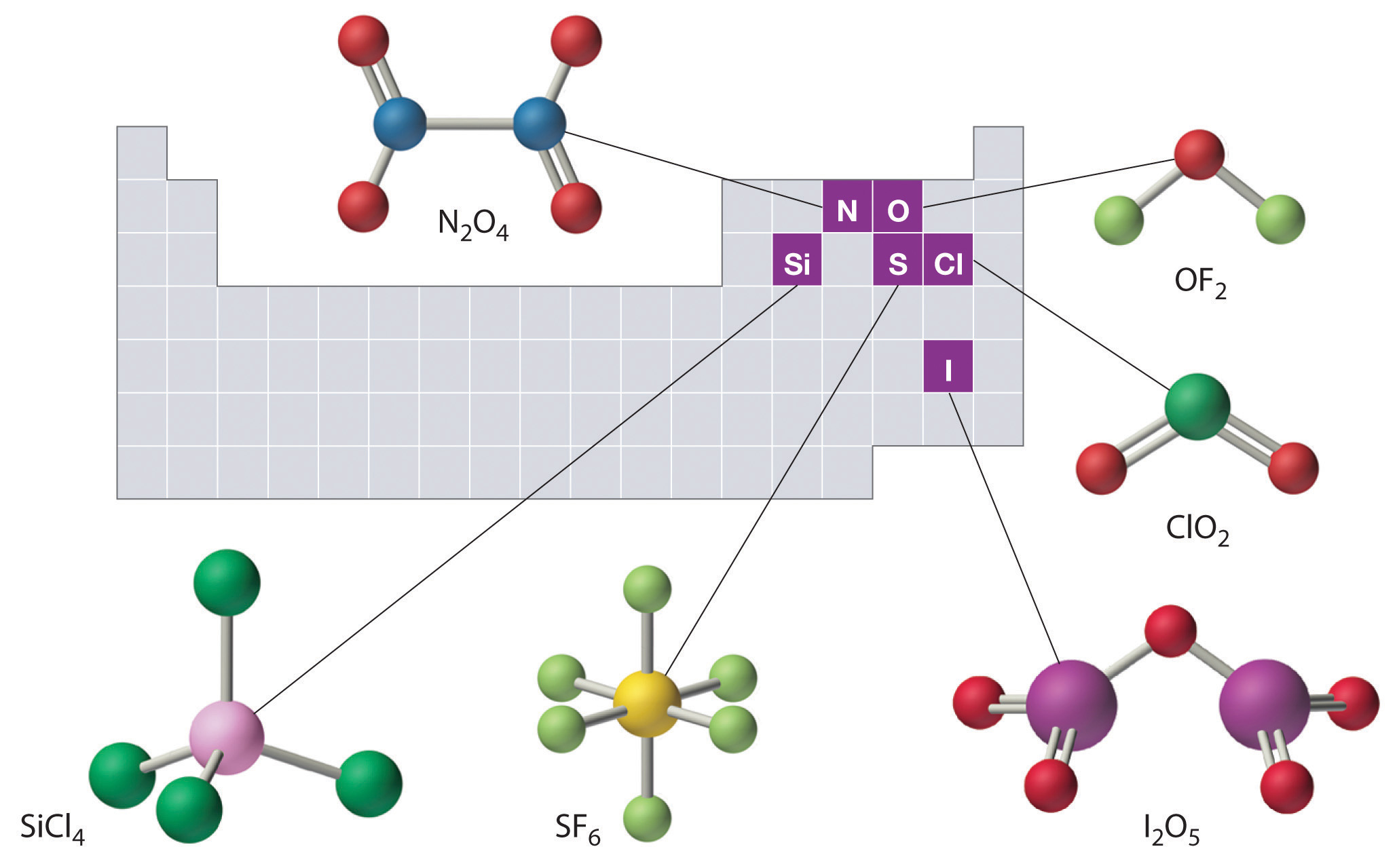
The structures of some of the compounds in Examples \(\PageIndex{3}\) and \(\PageIndex{4}\) are shown in Figure \(\PageIndex{2}\) along with the location of the “central atom” of each compound in the periodic table. It may seem that the compositions and structures of such compounds are entirely random, but this is not true. After mastering the material discussed later on this course, one is able to predict the compositions and structures of compounds of this type with a high degree of accuracy.
Nomenclature of Nonmetals: Nomenclature of Nonmetals, YouTube(opens in new window) [youtu.be] (opens in new window)
Summary
The composition of a compound is represented by an empirical or molecular formula, each consisting of at least one formula unit. Covalent inorganic compounds are named using a procedure similar to that used for ionic compounds, whereas hydrocarbons use a system based on the number of bonds between carbon atoms. Covalent inorganic compounds are named by a procedure similar to that used for ionic compounds, using prefixes to indicate the numbers of atoms in the molecular formula. An empirical formula gives the relative numbers of atoms of the elements in a compound, reduced to the lowest whole numbers. The formula unit is the absolute grouping represented by the empirical formula of a compound, either ionic or covalent. Empirical formulas are particularly useful for describing the composition of ionic compounds, which do not contain readily identifiable molecules. Some ionic compounds occur as hydrates, which contain specific ratios of loosely bound water molecules called waters of hydration.

