18.2: Group 1A Metals
- Page ID
- 60394
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Li, Na, K, Rb, and Cs are all group IA elements, also known as the alkali metals. The seventh member of the group, francium (Fr) is radioactive and so rare that only 20 atoms of Fr may exist on Earth at any given moment[1]. The term alkali is derived from an Arabic word meaning “ashes.” Compounds of potassium as well as other alkali metals were obtained from wood ashes by early chemists. All the alkali metals are soft and, except for Cs which is yellow, are silvery-gray in color.
Lithium, sodium, potassium, rubidium, and cesium have a great many other properties in common. All are solids at 0°C and melt below 200°C. Each has metallic properties such as good conduction of heat and electricity, malleability (the ability to be hammered into sheets), and ductility (the ability to be drawn into wires). The high thermal (heat) conductivity and the relatively low melting point (for a metal) of sodium make it an ideal heat-transfer fluid. It is used to cool certain types of nuclear reactors (liquid-metal fast breeder reactors, LMFBRs) and to cool the valves of high-powered automobile engines for this reason.
Some general properties of the alkali metals are summarized in the table below. All these metal atoms contain a singles electron outside a noble-gas configuration, and so the valence electron is-well shielded from nuclear charge and the atomic radii are relatively large. The large volume of each atom results in a low density—small enough that Li, Na, and K float on water as they react with it.
| Element | Symbol | Electron Configuration | Usual Oxidation State | Atomic Radius/pm | Ionic (M+) Radius/pm |
|---|---|---|---|---|---|
| Lithium | Li | [He]2s1 | +1 | 122 | 60 |
| Sodium | Na | [Ne]3s1 | +1 | 157 | 95 |
| Potassium | K | [Ar]4s1 | +1 | 202 | 133 |
| Rubidium | Rb | [Kr]5s1 | +1 | 216 | 148 |
| Cesium | Cs | [Xe]6s1 | +1 | 235 | 169 |
| Symbol | Ionization Energy/MJ mol–1 | Density/ g cm–3 | Electronegativity | Melting Point (in °C) | |
|---|---|---|---|---|---|
| First | Second | ||||
| Li | 0.526 | 7.305 | 0.534 | 1.0 | 179 |
| Na | 0.502 | 4.569 | 0.97 | 0.9 | 98 |
| K | 0.425 | 3.058 | 0.86 | 0.8 | 64 |
| Rb | 0.409 | 2.638 | 1.52 | 0.8 | 39 |
| Cs | 0.382 | 2.430 | 1.87 | 0.7 | 28 |
The atoms do not have a strong attraction for the single valence electron, and so it is easily lost (small first ionization energy) to from a +1 ion. Because they readily donate electrons in this way, all the alkali metals are strong reducing agents. They are quite reactive, even reducing water.
Weak attraction for the valence electron also results in weak metallic bonding, because it is attraction among nuclei and numerous valence electrons that holds metal atoms together. Weak metallic bonding results in low melting points, especially for the larger atoms toward the bottom of the group. Cs, for example, melts just above room temperature. Weak metallic bonding also accounts for the fact that all these metals are rather soft.
That the chemistry of alkali metals is confined to the +1 oxidation state is confirmed by the large second-ionization energies. Removing the first electron from the large, diffuses orbital is easy, but removing a second electron from an octet in an M+ ion is much too difficult for any oxidizing agent to do.
Two other elements are found in group IA. Hydrogen, although many of its compounds have formulas similar to the alkali metals, is a nonmetal and is almost unique in its chemical behavior. Therefore it is not usually included in this group. Francium (Fr) is quite radioactive, and only small quantities are available for study; so it too is usually omitted. Its properties, however, appear to be similar to those of Cs and the other alkali metals.
Chemical Reactions and Compounds
The element lithium combines violently and spectacularly with water. Hydrogen gas is given off, which propels the the lithium metal across the water as it reacts. If the excess water is evaporated, the compound lithium hydroxide (LiOH) remains behind. LiOH is visualized by phenolphthalein indicator, which turns pink as LiOH, a base, is produced. Thus the equation for this reaction is
\[\text{2Li}(s) + \text{2H}_\text{2}\text{O}(l) \rightarrow \text{2LiOH}(aq) + \text{H}_\text{2}(g)\nonumber \]
The elements sodium, potassium, rubidium, and cesium also combine violently with water to form hydroxides. The equations for their reactions are
\[\text{2Na}(s) + \text{2H}_\text{2}\text{O}(l) \rightarrow \text{2NaOH}(aq) + \text{H}_\text{2} (g)\nonumber \]
\[\text{2K}(s) + \text{2H}_\text{2}\text{O}(l) \rightarrow \text{2KOH}(aq) + \text{H}_\text{2} (g)\nonumber \]
\[\text{2Rb}(s) + \text{2H}_\text{2}\text{O}(l) \rightarrow \text{2RbOH}(aq) + \text{H}_\text{2} (g)\nonumber \]
\[\text{2Cs}(s) + \text{2H}_\text{2}\text{O}(l) \rightarrow \text{2CsOH}(aq) + \text{H}_\text{2} (g)\nonumber \]
Since the alkali metals all react with water in the same way, a general equation may be written:
\[\text{2M}(s) + \text{2H}_\text{2}\text{O}(l) \rightarrow \text{2MOH}(aq) + \text{H}_\text{2} (g)\nonumber \]
with M = K, Li, Na, Rb, or Cs.
The symbol M represents any one of the five elements.
In addition to their behavior when added to water, the alkali metals react directly with many elements. All combine swiftly with oxygen in air to form white oxide:
\[\text{4M}(s) + \text{O}_2(g) \rightarrow \text{2M}_2 \text{O}(s)\nonumber \]
with M = Li, Na, K, Rb, or Cs
(Li2O is lithium oxide, Na2O is sodium oxide, etc.)
All except lithium react further to form yellow peroxides, M2O2:
\[\text{2M}_2 \text{O}(s) + \text{O}_2(g) \rightarrow \text{2M}_2 \text{O}_2(s)\nonumber \] M = Na, K, Rb, or Cs
(Na2O2 is sodium peroxide, etc.)
Potassium, rubidium, and cesium are sufficiently reactive that yellow superoxides (whose general formula is MO2) can be formed:
\[\text{2M}_2 \text{O}_2(s) + \text{O}_2(g) \rightarrow \text{2MO}_2(s)\nonumber \]
with M = K, Rb, or Cs
Unless the surface of a sample of an alkali metal is scraped clean, it will appear white or gray instead of having a silvery metallic luster. This is due to the oxide, peroxide, or superoxide coating that forms after a few seconds of exposure to air. The following movie shows how a freshly cut piece of lithium is shiny, but dulls to gray when exposed to oxygen in the air. The video also focuses on another important property of alkali metals: they are soft, and easy to cut, compared to other metals.
A dull gray oxidized cylinder of lithium metal is cut, revealing a shiny silvery surface. After 1 minute, the surface has dulled, and after 10 minutes, the cut surface has returned to the dull gray of the rest of the lithium metal. Since the alkali metal is lithium, the only reaction with oxygen that occurs is:
\[\text{4Li}(s) + \text{O}_2(g) \rightarrow \text{2Li}_2\text{O}(s)\nonumber \]
The alkali also combine directly with hydrogen gas to form compounds known as hydrides, MH:
\[\text{2M}(s) + \text{H}_2(g) \rightarrow \text{2MH}(s)\nonumber \]
with M = Li, Na, K, Rb, or Cs
They react with sulfur to form sulfides, M2S:
\[\text{2M}(s) + \text{S}(g) \rightarrow \text{M}_2\text{S}(s)\nonumber \]
with M = Li, Na, K, Rb, or Cs
These oxides, hydrides, hydroxides, and sulfides all dissolve in water to give basic solutions, and these compounds are among the strong bases.
The peroxides and superoxides formed when the heavier alkali metals react with O2 also dissolve to give basic solutions:
\[\text{2NaO}_2(s) + \text{2H}_2\text{O}(l) \rightarrow \text{4Na}^{+}(aq) + \text{4OH}^{-}(aq) + \text{O}_2(g)\nonumber \]
\[\text{4K}_2\text{O}(s) + \text{2H}_2\text{O}(l) \rightarrow \text{4K}^{+}+ \text{4OH}^{-} + \text{3O}_2(g)\nonumber \]
Both of the latter equations describe redox as well as acid-base processes, as you can confirm by assigning oxidation numbers. The peroxide and superoxide ions contain O atoms in the unusual (for O) –1 and –½ oxidation states:
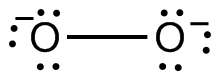
Therefore disproportionation (simultaneous oxidation and reduction) of O22– or O2– to the more common oxidation states of 0 (in O2) and –2 (in OH–) is possible.
The alkali metals also react directly with the halogens, for instance with chlorine, forming chlorides,
\[\text{2M}(s) + \text{Cl}_2(g) \rightarrow \text{2MCl}(s)\nonumber \] M = Li, Na, K, Rb, or Cs
Below is an example of the reaction of Na with Cl2
A piece of sodium metal is added to a flask containing chlorine gas. Initially no reaction takes place, but when a drop of water is added, sodium and chlorine react, violently flaring up and producing so much heat that sand is needed in the bottom of the flask to absorb the heat and prevent the glass from cracking. This equation for this reaction is:
\[\text{2Na}(s) + \text{Cl}_2(g) \rightarrow \text{2NaCl}(s)\nonumber \]
with fluorine to form fluorides, MF:
\[\text{2M}(s) + \text{F}_2(g) \rightarrow \text{2MF}(s)\nonumber \] M = Li, Na, K, Rb, or Cs
and with bromine to form bromides, MBr:
\[\text{2M}(s) + \text{Br}_2(g) \rightarrow \text{2MBr}(s)\nonumber \] M = Li, Na, K, Rb, or Cs
Below is an example of K reacting with Br2
In this video, potassium, which is stored in inert mineral oil due to its high reactivity, is placed in a beaker of liquid bromine after the protective layer of mineral oil has been removed. The potassium reacts explosively with the bromine. The container is covered during the whole process to prevent reactants and products from entering the environment. The chemical equation for this reaction is:
\[\text{2K}(s) + \text{Br}_2(g) \rightarrow \text{2KBr}(s)\nonumber \]
Sodium and potassium are quite abundant, ranking sixth and seventh among all elements in the earth’s crust, but the other alkali metals are rare. Sodium and potassium ions are components of numerous silicate crystal lattices seen in the Earth's crust, but since most of their compounds are water soluble, they are also important constituents of seawater and underground deposits of brine. Sodium chloride obtained from such brines is the chief commercial source of sodium, while potassium can be obtained from the ores sylvite (KCl) or carnallite (KCl•MgCl2•6H2O).
Both sodium (Na+) and potassium (K+) ions are essential to living systems. Na+ is the main cation in fluids surrounding the cells, while K+is most important inside the cells. Na+ plays a role in muscle contraction, and both K+ and Na+ play a role in transmitting nerve impulses. K is more important than Na in plants, and it is one of three elements (K, P, N) which must be supplied in fertilizer to maintain high crop yields. K is especially abundant in trees—wood ashes from kitchen fires (potash) were the major source of this element as recently as a century ago, and they still make good fertilizer for your garden. Wood ashes contain a mixture of potassium oxide and potassium carbonate, the latter formed by combination of K2O with CO2 produced when C in the wood combines with O2:
\[\text{K}_2\text{O} + \text{CO}_2 \rightarrow \text{K}_2\text{CO}_3\nonumber \]
Na compounds are obtained commercially from brine or from seawater. When an electrical current is passed through an NaCl solution (a process called electrolysis), Cl2(g), H2(g), and a concentrated solution of NaOH (caustic soda or lye) are obtained:
\[\text{Na}^{+}(aq) + \text{2Cl}^{-}(aq) + \text{2H}_2\text{O}(l) \xrightarrow{\text{electrolysis}} \text{Cl}_2(g) + \text{H}_2(g) + \text{Na}^{+}(aq) + \text{2OH}^{-}(aq)\nonumber \]
This process is described in more detail in the section on electrochemical cells, but you can see from the equation that the electrical current oxidizes Cl– to Cl2 and reduces H2O to H2. NaOH(aq) is used as a strong base in numerous industrial processes to make soap, rayon, cellophane, paper, dyes, and many other products. Lye is also used in home drain cleaners. It must be handled with care because it is strongly basic, highly caustic, and can severely burn the skin. A second important industrial use of brine is the Solvay process:
\[\text{CO}_2(g) + \text{NH}_3(aq) + \text{Na}^{+}(aq) + \text{Cl}^{-}(aq) + \text{H}_2\text{O}(l) \rightarrow \text{NaHCO}_3(s) + \text{NH}_4^{+}(aq) + \text{Cl}^{-}(aq)\nonumber \]
The Solvay process is an acid-base reaction combined with a precipitation. The acid anhydride, CO2, reacts with H2O to produce H2CO3. This weak acid donates a proton to NH3, yielding NH4+ and HCO3–, and the latter ion precipitates with Na+. The weakly basic sodium hydrogen carbonate produced by the Solvay process can be purified for use as an antacid (bicarbonate of soda), but most of it is converted to sodium carbonate (soda ash) by heating:
\[\text{2NaHCO}_3(s) \xrightarrow{\Delta } \text{Na}_2\text{CO}_3(s) + \text{H}_2\text{O}(g) + \text{CO}_2(g)\nonumber \]
(The Δ in this equation indicates heating of the reactant.) Sodium carbonate (Na2CO3) is used in manufacturing glass and paper, and in some detergents. The carbonate ion is a rather strong base, however, and detergents containing Na2CO3 (washing soda) have resulted in severe chemical burns to some small children who, out of curiosity, have eaten them.

