6.E: Electronic Structure (Exercises)
- Page ID
- 43480
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)6.1: The Wave Nature of Light
Conceptual Problems
- What are the characteristics of a wave? What is the relationship between electromagnetic radiation and wave energy?
- At constant wavelength, what effect does increasing the frequency of a wave have on its speed? its amplitude?
- List the following forms of electromagnetic radiation in order of increasing wavelength: x-rays, radio waves, infrared waves, microwaves, ultraviolet waves, visible waves, and gamma rays. List them in order of increasing frequency. Which has the highest energy?
- A large industry is centered on developing skin-care products, such as suntan lotions and cosmetics, that cannot be penetrated by ultraviolet radiation. How does the wavelength of visible light compare with the wavelength of ultraviolet light? How does the energy of visible light compare with the energy of ultraviolet light? Why is this industry focused on blocking ultraviolet light rather than visible light?
Numerical Problems
-
The human eye is sensitive to what fraction of the electromagnetic spectrum, assuming a typical spectral range of 104 to 1020 Hz? If we came from the planet Krypton and had x-ray vision (i.e., if our eyes were sensitive to x-rays in addition to visible light), how would this fraction be changed?
-
What is the frequency in megahertz corresponding to each wavelength?
- 755 m
- 6.73 nm
- 1.77 × 103 km
- 9.88 Å
- 3.7 × 10−10 m
-
What is the frequency in megahertz corresponding to each wavelength?
- 5.8 × 10−7 m
- 2.3 Å
- 8.6 × 107 m
- 6.2 mm
- 3.7 nm
-
Line spectra are also observed for molecular species. Given the following characteristic wavelengths for each species, identify the spectral region (ultraviolet, visible, etc.) in which the following line spectra will occur. Given 1.00 mol of each compound and the wavelength of absorbed or emitted light, how much energy does this correspond to?
- NH3, 1.0 × 10−2 m
- CH3CH2OH, 9.0 μm
- Mo atom, 7.1 Å
-
What is the speed of a wave in meters per second that has a wavelength of 1250 m and a frequency of 2.36 × 105 s−1?
-
A wave travels at 3.70 m/s with a frequency of 4.599 × 107 Hz and an amplitude of 1.0 m. What is its wavelength in nanometers?
-
An AM radio station broadcasts with a wavelength of 248.0 m. What is the broadcast frequency of the station in kilohertz? An AM station has a broadcast range of 92.6 MHz. What is the corresponding wavelength range in meters for this reception?
-
An FM radio station broadcasts with a wavelength of 3.21 m. What is the broadcast frequency of the station in megahertz? An FM radio typically has a broadcast range of 82–112 MHz. What is the corresponding wavelength range in meters for this reception?
-
A microwave oven operates at a frequency of approximately 2450 MHz. What is the corresponding wavelength? Water, with its polar molecules, absorbs electromagnetic radiation primarily in the infrared portion of the spectrum. Given this fact, why are microwave ovens used for cooking food?
6.2: Quantized Energy and Photons
Conceptual Problems
- Describe the relationship between the energy of a photon and its frequency.
- How was the ultraviolet catastrophe explained?
- If electromagnetic radiation with a continuous range of frequencies above the threshold frequency of a metal is allowed to strike a metal surface, is the kinetic energy of the ejected electrons continuous or quantized? Explain your answer.
- The vibrational energy of a plucked guitar string is said to be quantized. What do we mean by this? Are the sounds emitted from the 88 keys on a piano also quantized?
- Which of the following exhibit quantized behavior: a human voice, the speed of a car, a harp, the colors of light, automobile tire sizes, waves from a speedboat?
Conceptual Answers
-
The energy of a photon is directly proportional to the frequency of the electromagnetic radiation.
-
-
-
-
Quantized: harp, tire size, speedboat waves; continuous: human voice, colors of light, car speed.
Numerical Problems
-
What is the energy of a photon of light with each wavelength? To which region of the electromagnetic spectrum does each wavelength belong?
- 4.33 × 105 m
- 0.065 nm
- 786 pm
-
How much energy is contained in each of the following? To which region of the electromagnetic spectrum does each wavelength belong?
- 250 photons with a wavelength of 3.0 m
- 4.2 × 106 photons with a wavelength of 92 μm
- 1.78 × 1022 photons with a wavelength of 2.1 Å
-
A 6.023 x 1023 photons are found to have an energy of 225 kJ. What is the wavelength of the radiation?
-
Use the data in Table 2.1.1 to calculate how much more energetic a single gamma-ray photon is than a radio-wave photon. How many photons from a radio source operating at a frequency of 8 × 105 Hz would be required to provide the same amount of energy as a single gamma-ray photon with a frequency of 3 × 1019 Hz?
-
Use the data in Table 2.1.1 to calculate how much more energetic a single x-ray photon is than a photon of ultraviolet light.
-
A radio station has a transmitter that broadcasts at a frequency of 100.7 MHz with a power output of 50 kW. Given that 1 W = 1 J/s, how many photons are emitted by the transmitter each second?
Numerical Answers
-
- 4.59 × 10−31 J/photon, radio
- 3.1 × 10−15 J/photon, gamma ray
- 2.53 × 10−16 J/photon, gamma ray
-
-
532 nm
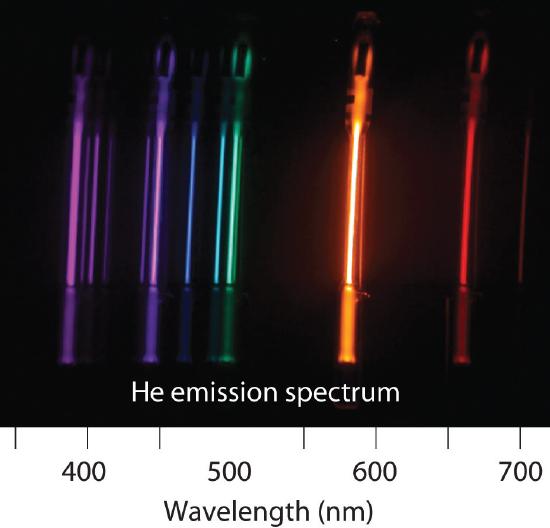
6.3: Line Spectra and the Bohr Model
Conceptual Problems
-
Is the spectrum of the light emitted by isolated atoms of an element discrete or continuous? How do these spectra differ from those obtained by heating a bulk sample of a solid element? Explain your answers.
-
Explain why each element has a characteristic emission and absorption spectra. If spectral emissions had been found to be continuous rather than discrete, what would have been the implications for Bohr’s model of the atom?
-
Explain the differences between a ground state and an excited state. Describe what happens in the spectrum of a species when an electron moves from a ground state to an excited state. What happens in the spectrum when the electron falls from an excited state to a ground state?
-
What phenomenon causes a neon sign to have a characteristic color? If the emission spectrum of an element is constant, why do some neon signs have more than one color?
-
How is light from a laser different from the light emitted by a light source such as a light bulb? Describe how a laser produces light.
Numerical Problems
-
Using a Bohr model and the transition from n = 2 to n = 3 in an atom with a single electron, describe the mathematical relationship between an emission spectrum and an absorption spectrum. What is the energy of this transition? What does the sign of the energy value represent in this case? What range of light is associated with this transition?
-
If a hydrogen atom is excited from an n = 1 state to an n = 3 state, how much energy does this correspond to? Is this an absorption or an emission? What is the wavelength of the photon involved in this process? To what region of the electromagnetic spectrum does this correspond?
-
The hydrogen atom emits a photon with a 486 nm wavelength, corresponding to an electron decaying from the n = 4 level to which level? What is the color of the emission?
-
An electron in a hydrogen atom can decay from the n = 3 level to n = 2 level. What is the color of the emitted light? What is the energy of this transition?
-
Calculate the wavelength and energy of the photon that gives rise to the third line in order of increasing energy in the Lyman series in the emission spectrum of hydrogen. In what region of the spectrum does this wavelength occur? Describe qualitatively what the absorption spectrum looks like.
-
The wavelength of one of the lines in the Lyman series of hydrogen is 121 nm. In what region of the spectrum does this occur? To which electronic transition does this correspond?
-
Removing an electron from solid potassium requires 222 kJ/mol. Would you expect to observe a photoelectric effect for potassium using a photon of blue light (λ = 485 nm)? What is the longest wavelength of energy capable of ejecting an electron from potassium? What is the corresponding color of light of this wavelength?
-
The binding energy of an electron is the energy needed to remove an electron from its lowest energy state. According to Bohr’s postulates, calculate the binding energy of an electron in a hydrogen atom. There are 6.02 x 1023 atoms in 1g of hydrogen atoms What wavelength in nanometers is required to remove such an electron from one hydrogen atom?
-
As a radio astronomer, you have observed spectral lines for hydrogen corresponding to a state with n = 320, and you would like to produce these lines in the laboratory. Is this feasible? Why or why not?
Numerical Answers
-
656 nm; red light
-
n = 2, blue-green light
-
97.2 nm, 2.04 × 10−18 J/photon, ultraviolet light, absorption spectrum is a single dark line at a wavelength of 97.2 nm
-
Violet: 390 nm, 307 kJ/mol photons; Blue-purple: 440 nm, 272 kJ/mol photons; Blue-green: 500 nm, 239 kJ/mol photons; Orange: 580 nm, 206 kJ/mol photons; Red: 650 nm, 184 kJ/mol photons
-
1313 kJ/mol, λ ≤ 91.1 nm
6.4: The Wave Behavior of Matter
Conceptual Problems
-
Explain what is meant by each term and illustrate with a sketch:
- standing wave
- fundamental
- overtone
- node
-
How does Einstein’s theory of relativity illustrate the wave–particle duality of light? What properties of light can be explained by a wave model? What properties can be explained by a particle model?
-
In the modern theory of the electronic structure of the atom, which of de Broglie’s ideas have been retained? Which proved to be incorrect?
-
According to Bohr, what is the relationship between an atomic orbit and the energy of an electron in that orbit? Is Bohr’s model of the atom consistent with Heisenberg’s uncertainty principle? Explain your answer.
Numerical Problems
-
How much heat is generated by shining a carbon dioxide laser with a wavelength of 1.065 μm on a 68.95 kg sample of water if 1.000 mol of photons is absorbed and converted to heat? Is this enough heat to raise the temperature of the water by 4°C?
-
Show the mathematical relationship between energy and mass and between wavelength and mass. What is the effect of doubling the
- mass of an object on its energy?
- mass of an object on its wavelength?
- frequency on its mass?
-
What is the de Broglie wavelength of a 39 g bullet traveling at 1020 m/s ± 10 m/s? What is the minimum uncertainty in the bullet’s position?
-
What is the de Broglie wavelength of a 6800 tn aircraft carrier traveling at 18 ± 0.1 knots (1 knot = 1.15 mi/h)? What is the minimum uncertainty in its position?
-
Calculate the mass of a particle if it is traveling at 2.2 × 106 m/s and has a frequency of 6.67 × 107 Hz. If the uncertainty in the velocity is known to be 0.1%, what is the minimum uncertainty in the position of the particle?
-
Determine the wavelength of a 2800 lb automobile traveling at 80 mi/h ± 3%. How does this compare with the diameter of the nucleus of an atom? You are standing 3 in. from the edge of the highway. What is the minimum uncertainty in the position of the automobile in inches?
Numerical Answers
-
E = 112.3 kJ, ΔT = 0.3893°C, over ten times more light is needed for a 4.0°C increase in temperature
-
-
1.7 × 10−35 m, uncertainty in position is ≥ 1.4 × 10−34 m
-
-
9.1 × 10−39 kg, uncertainty in position ≥ 2.6 m
6.5: Quantum Mechanics and Atomic Orbitals
Conceptual Problems
-
Why does an electron in an orbital with n = 1 in a hydrogen atom have lower energy than a free electron (n = ∞)?
-
What four variables are required to fully describe the position of any object in space? In quantum mechanics, one of these variables is not explicitly considered. Which one and why?
-
Chemists generally refer to the square of the wave function rather than to the wave function itself. Why?
-
Orbital energies of species with only one electron are defined by only one quantum number. Which one? In such a species, is the energy of an orbital with n = 2 greater than, less than, or equal to the energy of an orbital with n = 4? Justify your answer.
-
In each pair of subshells for a hydrogen atom, which has the higher energy? Give the principal and the azimuthal quantum number for each pair.
- 1s, 2p
- 2p, 2s
- 2s, 3s
- 3d, 4s
-
What is the relationship between the energy of an orbital and its average radius? If an electron made a transition from an orbital with an average radius of 846.4 pm to an orbital with an average radius of 476.1 pm, would an emission spectrum or an absorption spectrum be produced? Why?
-
In making a transition from an orbital with a principal quantum number of 4 to an orbital with a principal quantum number of 7, does the electron of a hydrogen atom emit or absorb a photon of energy? What would be the energy of the photon? To what region of the electromagnetic spectrum does this energy correspond?
-
What quantum number defines each of the following?
- the overall shape of an orbital
- the orientation of an electron with respect to a magnetic field
- the orientation of an orbital in space
- the average energy and distance of an electron from the nucleus
-
In an attempt to explain the properties of the elements, Niels Bohr initially proposed electronic structures for several elements with orbits holding a certain number of electrons, some of which are in the following table:
Element Number of Electrons Electrons in orbits with n = 4 3 2 1 H 1 1 He 2 2 Ne 10 8 2 Ar 18 8 8 2 Li 3 1 2 Na 11 1 8 2 K 19 1 8 8 2 Be 4 2 2 - Draw the electron configuration of each atom based only on the information given in the table. What are the differences between Bohr’s initially proposed structures and those accepted today?
- Using Bohr’s model, what are the implications for the reactivity of each element?
- Give the actual electron configuration of each element in the table.
Numerical Problems
-
How many subshells are possible for n = 3? What are they?
-
How many subshells are possible for n = 5? What are they?
-
What value of l corresponds to a d subshell? How many orbitals are in this subshell?
-
What value of l corresponds to an f subshell? How many orbitals are in this subshell?
-
State the number of orbitals and electrons that can occupy each subshell.
- 2s
- 3p
- 4d
- 6f
-
State the number of orbitals and electrons that can occupy each subshell.
- 1s
- 4p
- 5d
- 4f
-
How many orbitals and subshells are found within the principal shell n = 6? How do these orbital energies compare with those for n = 4?
-
How many nodes would you expect a 4p orbital to have? A 5s orbital?
-
A p orbital is found to have one node in addition to the nodal plane that bisects the lobes. What would you predict to be the value of n? If an s orbital has two nodes, what is the value of n?
Numerical Answers
-
Three subshells, with l = 0 (s), l = 1 (p), and l = 2 (d).
-
-
A d subshell has l = 2 and contains 5 orbitals.
-
-
- 2 electrons; 1 orbital
- 6 electrons; 3 orbitals
- 10 electrons; 5 orbitals
- 14 electrons; 7 orbitals
-
-
A principal shell with n = 6 contains six subshells, with l = 0, 1, 2, 3, 4, and 5, respectively. These subshells contain 1, 3, 5, 7, 9, and 11 orbitals, respectively, for a total of 36 orbitals. The energies of the orbitals with n = 6 are higher than those of the corresponding orbitals with the same value of l for n = 4.
6.7: Many-Electron Atoms
Conceptual Problems
-
A set of four quantum numbers specifies each wave function. What information is given by each quantum number? What does the specified wave function describe?
-
List two pieces of evidence to support the statement that electrons have a spin.
-
The periodic table is divided into blocks. Identify each block and explain the principle behind the divisions. Which quantum number distinguishes the horizontal rows?
-
Identify the element with each ground state electron configuration.
- [He]2s22p3
- [Ar]4s23d1
- [Kr]5s24d105p3
- [Xe]6s24f 6
-
Identify the element with each ground state electron configuration.
- [He]2s22p1
- [Ar]4s23d8
- [Kr]5s24d105p4
- [Xe]6s2
-
Propose an explanation as to why the noble gases are inert.
Numerical Problems
-
How many magnetic quantum numbers are possible for a 4p subshell? A 3d subshell? How many orbitals are in these subshells?
-
How many magnetic quantum numbers are possible for a 6s subshell? A 4f subshell? How many orbitals does each subshell contain?
-
If l = 2 and ml = 2, give all the allowed combinations of the four quantum numbers (n, l, ml, ms) for electrons in the corresponding 3d subshell.
-
Give all the allowed combinations of the four quantum numbers (n, l, ml, ms) for electrons in a 4d subshell. How many electrons can the 4d orbital accommodate? How would this differ from a situation in which there were only three quantum numbers (n, l, m)?
-
Given the following sets of quantum numbers (n, l, ml, ms), identify each principal shell and subshell.
- 1, 0, 0, ½
- 2, 1, 0, ½
- 3, 2, 0, ½
- 4, 3, 3, ½
-
Is each set of quantum numbers allowed? Explain your answers.
- n = 2; l = 1; ml = 2; ms = +½
- n = 3, l = 0; ml = −1; ms = −½
- n = 2; l = 2; ml = 1; ms = +½
- n = 3; l = 2; ml = 2; ms = +½
-
List the set of quantum numbers for each electron in the valence shell of each element.
- beryllium
- xenon
- lithium
- fluorine
-
List the set of quantum numbers for each electron in the valence shell of each element.
- carbon
- magnesium
- bromine
- sulfur
-
Sketch the shape of the periodic table if there were three possible values of ms for each electron (+½, −½, and 0); assume that the Pauli principle is still valid.
-
Predict the shape of the periodic table if eight electrons could occupy the p subshell.
-
If the electron could only have spin +½, what would the periodic table look like?
-
If three electrons could occupy each s orbital, what would be the electron configuration of each species?
- sodium
- titanium
- fluorine
- calcium
-
If Hund’s rule were not followed and maximum pairing occurred, how many unpaired electrons would each species have? How do these numbers compare with the number found using Hund’s rule?
- phosphorus
- iodine
- manganese
-
Write the electron configuration for each element in the ground state.
- aluminum
- calcium
- sulfur
- tin
- nickel
- tungsten
- neodymium
- americium
-
Write the electron configuration for each element in the ground state.
- boron
- rubidium
- bromine
- germanium
- vanadium
- palladium
- bismuth
- europium
-
Give the complete electron configuration for each element.
- magnesium
- potassium
- titanium
- selenium
- iodine
- uranium
- germanium
-
Give the complete electron configuration for each element.
- tin
- copper
- fluorine
- hydrogen
- thorium
- yttrium
- bismuth
-
Write the valence electron configuration for each element:
- samarium
- praseodymium
- boron
- cobalt
-
Using the Pauli exclusion principle and Hund’s rule, draw valence orbital diagrams for each element.
- barium
- neodymium
- iodine
-
Using the Pauli exclusion principle and Hund’s rule, draw valence orbital diagrams for each element.
- chlorine
- silicon
- scandium
-
How many unpaired electrons does each species contain?
- lead
- cesium
- copper
- silicon
- selenium
-
How many unpaired electrons does each species contain?
- helium
- oxygen
- bismuth
- silver
- boron
-
For each element, give the complete electron configuration, draw the valence electron configuration, and give the number of unpaired electrons present.
- lithium
- magnesium
- silicon
- cesium
- lead
-
Use an orbital diagram to illustrate the aufbau principle, the Pauli exclusion principle, and Hund’s rule for each element.
- carbon
- sulfur
Numerical Answers
-
For a 4p subshell, n = 4 and l = 1. The allowed values of the magnetic quantum number, ml, are therefore +1, 0, −1, corresponding to three 4p orbitals. For a 3d subshell, n = 3 and l = 2. The allowed values of the magnetic quantum number, ml, are therefore +2, +1, 0, −1, −2, corresponding to five 3d orbitals.