4.E: Aqueous Reactions (Exercises)
- Page ID
- 43475
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)4.1: General Properties of Aqueous Solutions
Conceptual Problems
-
What are the advantages to carrying out a reaction in solution rather than simply mixing the pure reactants?
-
What types of compounds dissolve in polar solvents?
-
Describe the charge distribution in liquid water. How does this distribution affect its physical properties?
-
Must a molecule have an asymmetric charge distribution to be polar? Explain your answer.
-
Why are many ionic substances soluble in water?
-
Explain the phrase like dissolves like.
-
What kinds of covalent compounds are soluble in water?
-
Why do most aromatic hydrocarbons have only limited solubility in water? Would you expect their solubility to be higher, lower, or the same in ethanol compared with water? Why?
-
Predict whether each compound will dissolve in water and explain why.
- toluene
- acetic acid
- sodium acetate
- butanol
- pentanoic acid
-
Predict whether each compound will dissolve in water and explain why.
- ammonium chloride
- 2-propanol
- heptane
- potassium dichromate
- 2-octanol
-
Given water and toluene, predict which is the better solvent for each compound and explain your reasoning.
- sodium cyanide
- benzene
- acetic acid
- sodium ethoxide (CH3CH2ONa)
-
Of water and toluene, predict which is the better solvent for each compound and explain your reasoning.
- t-butanol
- calcium chloride
- sucrose
- cyclohexene
-
Compound A is divided into three equal samples. The first sample does not dissolve in water, the second sample dissolves only slightly in ethanol, and the third sample dissolves completely in toluene. What does this suggest about the polarity of A?
-
You are given a mixture of three solid compounds—A, B, and C—and are told that A is a polar compound, B is slightly polar, and C is nonpolar. Suggest a method for separating these three compounds.
-
A laboratory technician is given a sample that contains only sodium chloride, sucrose, and cyclodecanone (a ketone). You must tell the technician how to separate these three compounds from the mixture. What would you suggest?
-
Many over-the-counter drugs are sold as ethanol/water solutions rather than as purely aqueous solutions. Give a plausible reason for this practice.
-
What distinguishes a weak electrolyte from a strong electrolyte?
-
Which organic groups result in aqueous solutions that conduct electricity?
-
It is considered highly dangerous to splash barefoot in puddles during a lightning storm. Why?
-
Which solution(s) would you expect to conduct electricity well? Explain your reasoning.
- an aqueous solution of sodium chloride
- a solution of ethanol in water
- a solution of calcium chloride in water
- a solution of sucrose in water
-
Which solution(s) would you expect to conduct electricity well? Explain your reasoning.
- an aqueous solution of acetic acid
- an aqueous solution of potassium hydroxide
- a solution of ethylene glycol in water
- a solution of ammonium chloride in water
-
Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in an aqueous solution? Explain your reasoning.
- potassium hydroxide
- ammonia
- calcium chloride
- butanoic acid
-
Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in an aqueous solution? Explain your reasoning.
- magnesium hydroxide
- butanol
- ammonium bromide
- pentanoic acid
-
Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in aqueous solution? Explain your reasoning.
- H2SO4
- diethylamine
- 2-propanol
- ammonium chloride
- propanoic acid
Conceptual Answers
-
Ionic compounds such as NaCl are held together by electrostatic interactions between oppositely charged ions in the highly ordered solid. When an ionic compound dissolves in water, the partially negatively charged oxygen atoms of the H2O molecules surround the cations, and the partially positively charged hydrogen atoms in H2O surround the anions. The favorable electrostatic interactions between water and the ions compensate for the loss of the electrostatic interactions between ions in the solid.
-
- Because toluene is an aromatic hydrocarbon that lacks polar groups, it is unlikely to form a homogenous solution in water.
- Acetic acid contains a carboxylic acid group attached to a small alkyl group (a methyl group). Consequently, the polar characteristics of the carboxylic acid group will be dominant, and acetic acid will form a homogenous solution with water.
- Because most sodium salts are soluble, sodium acetate should form a homogenous solution with water.
- Like all alcohols, butanol contains an −OH group that can interact well with water. The alkyl group is rather large, consisting of a 4-carbon chain. In this case, the nonpolar character of the alkyl group is likely to be as important as the polar character of the –OH, decreasing the likelihood that butanol will form a homogeneous solution with water.
- Like acetic acid, pentanoic acid is a carboxylic acid. Unlike acetic acid, however, the alkyl group is rather large, consisting of a 4-carbon chain as in butanol. As with butanol, the nonpolar character of the alkyl group is likely to be as important as the polar character of the carboxylic acid group, making it unlikely that pentanoic acid will form a homogeneous solution with water. (In fact, the solubility of both butanol and pentanoic acid in water is quite low, only about 3 g per 100 g water at 25°C.)
-
An electrolyte is any compound that can form ions when it dissolves in water. When a strong electrolyte dissolves in water, it dissociates completely to give the constituent ions. In contrast, when a weak electrolyte dissolves in water, it produces relatively few ions in solution.
4.2: Precipitation Reactions
Conceptual Problems
-
What information can be obtained from a complete ionic equation that cannot be obtained from the overall chemical equation?
-
Predict whether mixing each pair of solutions will result in the formation of a precipitate. If so, identify the precipitate.
- FeCl2(aq) + Na2S(aq)
- NaOH(aq) + H3PO4(aq)
- ZnCl2(aq) + (NH4)2S(aq)
-
Predict whether mixing each pair of solutions will result in the formation of a precipitate. If so, identify the precipitate.
- KOH(aq) + H3PO4(aq)
- K2CO3(aq) + BaCl2(aq)
- Ba(NO3)2(aq) + Na2SO4(aq)
-
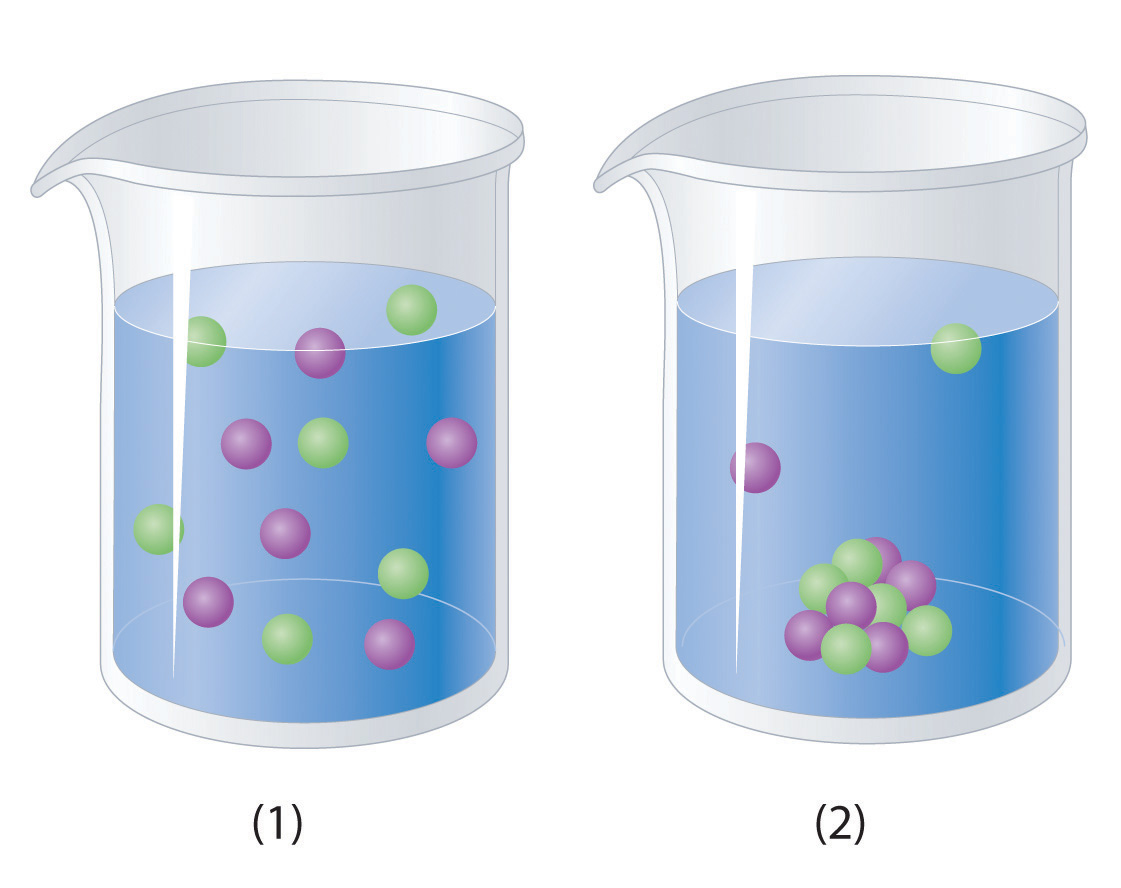
Which representation best corresponds to an aqueous solution originally containing each of the following?
- 1 M NH4Cl
- 1 M NaO2CCH3
- 1 M NaOH + 1 M HCl
-
1 M Ba(OH)2 + 1 M H2SO4
-
Which representation in Problem 3 best corresponds to an aqueous solution originally containing each of the following?
- 1 M CH3CO2H + 1 M NaOH
- 1 M NH3 + 1 M HCl
- 1 M Na2CO3 + 1 M H2SO4
- 1 M CaCl2 + 1 M H3PO4
Conceptual Answer
3
- 1
- 1
- 1
- 2
Numerical Problems
-
What mass of precipitate would you expect to obtain by mixing 250 mL of a solution containing 4.88 g of Na2CrO4 with 200 mL of a solution containing 3.84 g of AgNO3? What is the final nitrate ion concentration?
-
Adding 10.0 mL of a dilute solution of zinc nitrate to 246 mL of 2.00 M sodium sulfide produced 0.279 g of a precipitate. How many grams of zinc(II) nitrate and sodium sulfide were consumed to produce this quantity of product? What was the concentration of each ion in the original solutions? What is the concentration of the sulfide ion in solution after the precipitation reaction, assuming no further reaction?
Numerical Answer
-
3.75 g Ag2CrO4; 5.02 × 10−2 M nitrate
4.3: Acid-Base Reactions
Conceptual Problems
-
Why was it necessary to expand on the Arrhenius definition of an acid and a base? What specific point does the Brønsted–Lowry definition address?
-
State whether each compound is an acid, a base, or a salt.
- CaCO3
- NaHCO3
- H2SO4
- CaCl2
- Ba(OH)2
-
State whether each compound is an acid, a base, or a salt.
- NH3
- NH4Cl
- H2CO3
- CH3COOH
- NaOH
-
Classify each compound as a strong acid, a weak acid, a strong base, or a weak base in aqueous solution.
- sodium hydroxide
- acetic acid
- magnesium hydroxide
- tartaric acid
- sulfuric acid
- ammonia
- hydroxylamine (NH2OH)
- hydrocyanic acid
-
Decide whether each compound forms an aqueous solution that is strongly acidic, weakly acidic, strongly basic, or weakly basic.
- propanoic acid
- hydrobromic acid
- methylamine
- lithium hydroxide
- citric acid
- sodium acetate
- ammonium chloride
- barium hydroxide
-
What is the relationship between the strength of an acid and the strength of the conjugate base derived from that acid? Would you expect the CH3CO2− ion to be a strong base or a weak base? Why? Is the hydronium ion a strong acid or a weak acid? Explain your answer.
-
What are the products of an acid–base reaction? Under what circumstances is one of the products a gas?
-
Explain how an aqueous solution that is strongly basic can have a pH, which is a measure of the acidity of a solution.
Answer
-
- weakly acidic
- strongly acidic
- weakly basic
- strongly basic
- weakly acidic
- weakly basic
- weakly acidic
- strongly basic
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 3 (section 4.1 "Aqueous Solutions"0) before proceeding to the Numerical Problems.
-
Derive an equation to relate the hydrogen ion concentration to the molarity of a solution of a strong monoprotic acid.
-
Derive an equation to relate the hydroxide ion concentration to the molarity of a solution of
- a group I hydroxide.
- a group II hydroxide.
-
Given the following salts, identify the acid and the base in the neutralization reactions and then write the complete ionic equation:
- barium sulfate
- lithium nitrate
- sodium bromide
- calcium perchlorate
-
What is the pH of each solution?
- 5.8 × 10−3 mol of HNO3 in 257 mL of water
- 0.0079 mol of HI in 750 mL of water
- 0.011 mol of HClO4 in 500 mL of water
- 0.257 mol of HBr in 5.00 L of water
-
What is the hydrogen ion concentration of each substance in the indicated pH range?
- black coffee (pH 5.10)
- milk (pH 6.30–7.60)
- tomatoes (pH 4.00–4.40)
-
What is the hydrogen ion concentration of each substance in the indicated pH range?
- orange juice (pH 3–4)
- fresh egg white (pH 7.60–7.80)
- lemon juice (pH 2.20–2.40)
-
What is the pH of a solution prepared by diluting 25.00 mL of 0.879 M HCl to a volume of 555 mL?
-
Vinegar is primarily an aqueous solution of acetic acid. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. What is the concentration of commercial vinegar? If only 3.1% of the acetic acid dissociates to CH3CO2− and H+, what is the pH of the solution? (Assume the density of the solution is 1.00 g/mL.)
-
If a typical household cleanser is 0.50 M in strong base, what volume of 0.998 M strong monoprotic acid is needed to neutralize 50.0 mL of the cleanser?
-
A 25.00 mL sample of a 0.9005 M solution of HCl is diluted to 500.0 mL. What is the molarity of the final solution? How many milliliters of 0.223 M NaOH are needed to neutralize 25.00 mL of this final solution?
-
If 20.0 mL of 0.10 M NaOH are needed to neutralize 15.0 mL of gastric fluid, what is the molarity of HCl in the fluid? (Assume all the acidity is due to the presence of HCl.) What other base might be used instead of NaOH?
-
Malonic acid (C3H4O4) is a diprotic acid used in the manufacture of barbiturates. How many grams of malonic acid are in a 25.00 mL sample that requires 32.68 mL of 1.124 M KOH for complete neutralization to occur? Malonic acid is a dicarboxylic acid; propose a structure for malonic acid.
-
Describe how you would prepare 500 mL of a 1.00 M stock solution of HCl from an HCl solution that is 12.11 M. Using your stock solution, how would you prepare 500 mL of a solution that is 0.012 M in HCl?
-
Given a stock solution that is 8.52 M in HBr, describe how you would prepare a 500 mL solution with each concentration.
- 2.50 M
- 4.00 × 10−3 M
- 0.989 M
-
How many moles of solute are contained in each?
- 25.00 mL of 1.86 M NaOH
- 50.00 mL of 0.0898 M HCl
- 13.89 mL of 0.102 M HBr
-
A chemist needed a solution that was approximately 0.5 M in HCl but could measure only 10.00 mL samples into a 50.00 mL volumetric flask. Propose a method for preparing the solution. (Assume that concentrated HCl is 12.0 M.)
-
Write the balanced chemical equation for each reaction.
- perchloric acid with potassium hydroxide
- nitric acid with calcium hydroxide
-
Write the balanced chemical equation for each reaction.
- solid strontium hydroxide with hydrobromic acid
- aqueous sulfuric acid with solid sodium hydroxide
-
A neutralization reaction gives calcium nitrate as one of the two products. Identify the acid and the base in this reaction. What is the second product? If the product had been cesium iodide, what would have been the acid and the base? What is the complete ionic equation for each reaction?
Answers
-
[H3O+] = [HA] M
-
- H2SO4 and Ba(OH)2; 2H+ + SO42− + Ba2+ + 2OH− → 2H2O + Ba2+ + SO42−
- HNO3 and LiOH; H+ + NO3− + Li+ + OH− → H2O + Li+ + NO3−
- HBr and NaOH; H+ + Br− + Na+ + OH− → H2O + Na+ + Br−
- HClO4 and Ca(OH)2; 2H+ + 2ClO4− + Ca2+ + 2OH− → 2H2O + Ca2+ + 2ClO4−
-
- 7.9 × 10−6 M H+
- 5.0 × 10−7 to 2.5 × 10−8 M H+
- 1.0 × 10−4 to 4.0 × 10−5 M H+
-
pH = 1.402
-
25 mL
-
0.13 M HCl; magnesium carbonate, MgCO3, or aluminum hydroxide, Al(OH)3
-
1.00 M solution: dilute 41.20 mL of the concentrated solution to a final volume of 500 mL. 0.012 M solution: dilute 12.0 mL of the 1.00 M stock solution to a final volume of 500 mL.
-
- 4.65 × 10−2 mol NaOH
- 4.49 × 10−3 mol HCl
- 1.42 × 10−3 mol HBr
-
- HClO4 + KOH → KClO4 + H2O
- 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
-
The acid is nitric acid, and the base is calcium hydroxide. The other product is water.
\(2HNO_3 + Ca(OH)_2 \rightarrow Ca(NO_3)_2 + 2H_2O\)
The acid is hydroiodic acid, and the base is cesium hydroxide. The other product is water.
\( HI + CsOH \rightarrow CsI + H_2O \)
The complete ionic equations are
\( 2H^+ + 2NO_3^- + Ca^{2+} + 2OH^- \rightarrow Ca^{2+} + 2NO_3^- + H_2O\)
\( H^+ + I^- + Cs^+ + OH^- \rightarrow Cs^+ + I^- + H_2O \)
4.4: Oxidation-Reduction Reactions
Conceptual Problems
-
Which elements in the periodic table tend to be good oxidants? Which tend to be good reductants?
-
If two compounds are mixed, one containing an element that is a poor oxidant and one with an element that is a poor reductant, do you expect a redox reaction to occur? Explain your answer. What do you predict if one is a strong oxidant and the other is a weak reductant? Why?
-
In each redox reaction, determine which species is oxidized and which is reduced:
- Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
- Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l)
- BrO3−(aq) + 2MnO2(s) + H2O(l) → Br−(aq) + 2MnO4−(aq) + 2H+(aq)
-
Single-displacement reactions are a subset of redox reactions. In this subset, what is oxidized and what is reduced? Give an example of a redox reaction that is not a single-displacement reaction.
Conceptual Solutions
- In the periodic table, the trend for oxidation increases towards the top-right of the table, while the trend for reduction increases towards the bottom-left. That is to say, the nonmetals and metalloids (with the exception of Noble Gases) tend to be better oxidants, and alkali and transition metals tend to be better reductants.
-
If the compounds mixed are both poor oxidants and poor reductants, no redox reaction will occur. The poor oxidizing agent has a weak ability to gain electrons, while the reducing agent has a weak ability to donate electrons. Due to this, no electrons (or very few electrons) will be transferred, and no reaction will occur. If one compound is a strong oxidant and the other is a weak reductant, the reaction will progress, but not as far as it would have with a strong reductant. Some reductants, such as gold, are so weak that only the strongest of oxidants can oxidize them.
-
Which is Oxidized and Reduced:
- Zn is oxidized, and H is reduced.
- Cu is oxidized, and N is reduced.
- Mn is oxidized, and Br is reduced.
-
A single-displacement reaction occurs when one "free" element replaces another similar element in a compound. What is oxidized and what is reduced depends on the elements in question. For example, in the single-displacement reaction Zn(s)+2HCl(aq)→ZnCl2(aq)+H2(g), the free element Zinc is oxidized while Hydrogen is reduced. In Cl2(g)+2NaBr(aq)→2NaCl(aq)+Br2(l), on the other hand, the free element Chlorine is reduced while Bromine is oxidized.
-
An example of a non-single-displacement reaction is a double-displacement reaction, such as: 2KI(aq)+Pb(NO3)2(aq)→2KNO3(aq)+PbI2(s)
-
Numerical Problems
-
Balance each redox reaction under the conditions indicated.
- CuS(s) + NO3−(aq) → Cu2+(aq) + SO42−(aq) + NO(g); acidic solution
- Ag(s) + HS−(aq) + CrO42−(aq) → Ag2S(s) + Cr(OH)3(s); basic solution
- Zn(s) + H2O(l) → Zn2+(aq) + H2(g); acidic solution
- O2(g) + Sb(s) → H2O2(aq) + SbO2−(aq); basic solution
- UO22+(aq) + Te(s) → U4+(aq) + TeO42−(aq); acidic solution
-
Balance each redox reaction under the conditions indicated.
- MnO4−(aq) + S2O32−(aq) → Mn2+(aq) + SO42−(aq); acidic solution
- Fe2+(aq) + Cr2O72−(aq) → Fe3+(aq) + Cr3+(aq); acidic solution
- Fe(s) + CrO42−(aq) → Fe2O3(s) + Cr2O3(s); basic solution
- Cl2(aq) → ClO3−(aq) + Cl−(aq); acidic solution
- CO32−(aq) + N2H4(aq) → CO(g) + N2(g); basic solution
-
Using the activity series, predict what happens in each situation. If a reaction occurs, write the net ionic equation; then write the complete ionic equation for the reaction.
- Platinum wire is dipped in hydrochloric acid.
- Manganese metal is added to a solution of iron(II) chloride.
- Tin is heated with steam.
- Hydrogen gas is bubbled through a solution of lead(II) nitrate.
-
Using the activity series, predict what happens in each situation. If a reaction occurs, write the net ionic equation; then write the complete ionic equation for the reaction.
- A few drops of NiBr2 are dropped onto a piece of iron.
- A strip of zinc is placed into a solution of HCl.
- Copper is dipped into a solution of ZnCl2.
- A solution of silver nitrate is dropped onto an aluminum plate.
-
Dentists occasionally use metallic mixtures called amalgams for fillings. If an amalgam contains zinc, however, water can contaminate the amalgam as it is being manipulated, producing hydrogen gas under basic conditions. As the filling hardens, the gas can be released, causing pain and cracking the tooth. Write a balanced chemical equation for this reaction.
-
Copper metal readily dissolves in dilute aqueous nitric acid to form blue Cu2+(aq) and nitric oxide gas.
- What has been oxidized? What has been reduced?
- Balance the chemical equation.
-
Classify each reaction as an acid–base reaction, a precipitation reaction, or a redox reaction, or state if there is no reaction; then complete and balance the chemical equation:
- Pt2+(aq) + Ag(s) →
- HCN(aq) + NaOH(aq) →
- Fe(NO3)3(aq) + NaOH(aq) →
- CH4(g) + O2(g) →
-
Classify each reaction as an acid–base reaction, a precipitation reaction, or a redox reaction, or state if there is no reaction; then complete and balance the chemical equation:
- Zn(s) + HCl(aq) →
- HNO3(aq) + AlCl3(aq) →
- K2CrO4(aq) + Ba(NO3)2(aq) →
- Zn(s) + Ni2+(aq) → Zn2+(aq) + Ni(s)Conceptual
Numerical Solutions
- Balanced Redox Reactions:
- 3CuS(s) + 8NO3−(aq) + 8H+(aq) → 3Cu2+(aq) + 3SO42−(aq) + 8NO(g) + 4H2O(l)
- 6Ag(s) + 2CrO42-(aq) + 3HS-(aq) + 5H2O(l) → 3Ag2S(s) + 2Cr(OH)3(s) + 7OH-(aq)
- Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
- 2Sb(s) + 3O2(g) + 2H2O(l) + 2OH-(aq) → 2SbO2-(aq) + 3H2O2(aq)
- Te(s) + 3UO22+(aq) + 4H+(aq) → TeO42-(aq) + 3U4+(aq) + 2H2O(l)
Conceptual Problems
-
Which of the representations best corresponds to a 1 M aqueous solution of each compound? Justify your answers.
- NH3
- HF
- CH3CH2CH2OH
-
Na2SO4
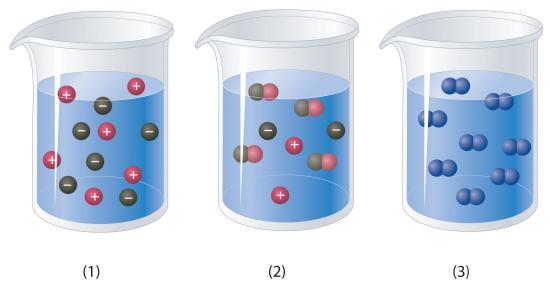
-
Which of the representations shown in Problem 1 best corresponds to a 1 M aqueous solution of each compound? Justify your answers.
- CH3CO2H
- NaCl
- Na2S
- Na3PO4
- acetaldehyde
-
Would you expect a 1.0 M solution of CaCl2 to be a better conductor of electricity than a 1.0 M solution of NaCl? Why or why not?
-
An alternative way to define the concentration of a solution is molality, abbreviated m. Molality is defined as the number of moles of solute in 1 kg of solvent. How is this different from molarity? Would you expect a 1 M solution of sucrose to be more or less concentrated than a 1 m solution of sucrose? Explain your answer.
-
What are the advantages of using solutions for quantitative calculations?
Conceptual Answer
-
Compound Corresponding Beaker Reasoning NH3 2 Weak base dissociates into both ions and molecules. HF 2 Weak acid dissociates into both ions and molecules. CH3CH2CH2OH 3 Covalent compound Na2SO4 1 Soluble ionic compound -
CH3CO2H 2 Weak acid dissociates into both ions and molecules. NaCl 1 Soluble ionic compound Na2S 1 Soluble ionic compound Na3PO4 1 Soluble ionic compound Acetaldehyde 1 Aldehydes with short chain lengths are miscible -
CaCl2 would be expected to be a better conductor than NaCl due to the fact that it contains more charges. The conductivity of a solution depends on the amount of mobile charges within it. While NaCl only has two charges (Na being + and Cl being -), CaCl2 contains three (Ca being 2+ and the two Cls being -). With more free charges when dissolved in the solution, CaCl2 would be a better conductor.
-
Assuming that the solvent is water, the solution with a Molarity (M) of 1 would be more concentrated than the solution with a Molality (m) of 1. Water is approximately 1 kg per liter, meaning that the solute is dissolved in 1 liter of water. This increases the amount of solution past 1 liter. Molarity, on the other hand, means that there is 1 liter of solution (that is, including both solute and solvent). Since in both cases there will be one mole, the 1M solution will be more concentrated than the 1m solution because there is less volume, but the same amount of solute.
-
If the amount of a substance required for a reaction is too small to be weighed accurately, the use of a solution of the substance, in which the solute is dispersed in a much larger mass of solvent, allows chemists to measure the quantity of the substance more accurately.
Numerical Problems
-
Calculate the number of grams of solute in 1.000 L of each solution.
- 0.2593 M NaBrO3
- 1.592 M KNO3
- 1.559 M acetic acid
- 0.943 M potassium iodate
-
Calculate the number of grams of solute in 1.000 L of each solution.
- 0.1065 M BaI2
- 1.135 M Na2SO4
- 1.428 M NH4Br
- 0.889 M sodium acetate
-
If all solutions contain the same solute, which solution contains the greater mass of solute?
- 1.40 L of a 0.334 M solution or 1.10 L of a 0.420 M solution
- 25.0 mL of a 0.134 M solution or 10.0 mL of a 0.295 M solution
- 250 mL of a 0.489 M solution or 150 mL of a 0.769 M solution
-
Complete the following table for 500 mL of solution.
Compound Mass (g) Moles Concentration (M) calcium sulfate 4.86 acetic acid 3.62 hydrogen iodide dihydrate 1.273 barium bromide 3.92 glucose 0.983 sodium acetate 2.42 -
What is the concentration of each species present in the following aqueous solutions?
- 0.489 mol of NiSO4 in 600 mL of solution
- 1.045 mol of magnesium bromide in 500 mL of solution
- 0.146 mol of glucose in 800 mL of solution
- 0.479 mol of CeCl3 in 700 mL of solution
-
What is the concentration of each species present in the following aqueous solutions?
- 0.324 mol of K2MoO4 in 250 mL of solution
- 0.528 mol of potassium formate in 300 mL of solution
- 0.477 mol of KClO3 in 900 mL of solution
- 0.378 mol of potassium iodide in 750 mL of solution
-
What is the molar concentration of each solution?
- 8.7 g of calcium bromide in 250 mL of solution
- 9.8 g of lithium sulfate in 300 mL of solution
- 12.4 g of sucrose (C12H22O11) in 750 mL of solution
- 14.2 g of iron(III) nitrate hexahydrate in 300 mL of solution
-
What is the molar concentration of each solution?
- 12.8 g of sodium hydrogen sulfate in 400 mL of solution
- 7.5 g of potassium hydrogen phosphate in 250 mL of solution
- 11.4 g of barium chloride in 350 mL of solution
- 4.3 g of tartaric acid (C4H6O6) in 250 mL of solution
-
Give the concentration of each reactant in the following equations, assuming 20.0 g of each and a solution volume of 250 mL for each reactant.
- BaCl2(aq) + Na2SO4(aq) →
- Ca(OH)2(aq) + H3PO4(aq) →
- Al(NO3)3(aq) + H2SO4(aq) →
- Pb(NO3)2(aq) + CuSO4(aq) →
- Al(CH3CO2)3(aq) + NaOH(aq) →
-
An experiment required 200.0 mL of a 0.330 M solution of Na2CrO4. A stock solution of Na2CrO4 containing 20.0% solute by mass with a density of 1.19 g/cm3 was used to prepare this solution. Describe how to prepare 200.0 mL of a 0.330 M solution of Na2CrO4 using the stock solution.
-
Calcium hypochlorite [Ca(OCl)2] is an effective disinfectant for clothing and bedding. If a solution has a Ca(OCl)2 concentration of 3.4 g per 100 mL of solution, what is the molarity of hypochlorite?
-
Phenol (C6H5OH) is often used as an antiseptic in mouthwashes and throat lozenges. If a mouthwash has a phenol concentration of 1.5 g per 100 mL of solution, what is the molarity of phenol?
-
If a tablet containing 100 mg of caffeine (C8H10N4O2) is dissolved in water to give 10.0 oz of solution, what is the molar concentration of caffeine in the solution?
-
A certain drug label carries instructions to add 10.0 mL of sterile water, stating that each milliliter of the resulting solution will contain 0.500 g of medication. If a patient has a prescribed dose of 900.0 mg, how many milliliters of the solution should be administered?
Numerical Answers
-
Grams of solute in 1.000 L of each solution:
- 0.2593 M NaBrO3
- 1.592 M KNO3
- 1.559 M acetic acid
- 0.943 M potassium iodate
- 0.2593 M NaBrO3
-
-
-
Compound Mass (g) Moles Concentration (M) calcium sulfate 4.86 0.0357 0.07 acetic acid 217.39 3.62 7.24 hydrogen iodide dihydrate 103.79 0.637 1.273 barium bromide 3.92 0.013 0.026 glucose 88.55 0.491 0.983 sodium acetate 198.52 2.42 4.84 -
Molar Concentration in each solution:
- 0.815M NiSO4
- 2.09M Magnesium Bromide
- 0.183M Glucose
- 0.684M CeCl3
-
Molar Concentration in each solution:
- 1.296M K2MoO4
- 1.760M Potassium Formate
- 0.530M KClO3
- 0.503M Potassium Iodide
-
Molar Concentration in each solution:
- 0.174M Calcium Bromide
- 0.297M Lithium Sulfate
- 0.048M Sucrose
- 0.135M Iron(III) Nitrate Hexahydrate
-
Molar Concentration in each solution:
- 0.267M Sodium Hydrogen Sulfate
- 0.172M Potassium Hydrogen Phosphate
- 0.156M Barium Chloride
- 0.115M Tartaric Acid
-
Concentration of Reactants in each solution:
- 0.192M of BaCl2, 0.282M of Na2SO4
- 0.541M of Ca(OH)2, 0.408M of H3PO4
- 0.188M of Al(NO3)3, 0.408M H2SO4(aq)
- 0.121M of Pb(NO3)2, 0.251M CuSO4
- 0.196M of Al(CH3CO2)3, 1M NaOH
-
-
0.4756 M ClO−
-
0.159M Phenol
-
1.74 × 10−3 M caffeine
-
1.8mL of solution
4.6: Solution Stoichiometry and Chemical Analysis
Conceptual Problems
-
The titration procedure is an application of the use of limiting reactants. Explain why this is so.
-
Explain how to determine the concentration of a substance using a titration.
-
Following are two graphs that illustrate how the pH of a solution varies during a titration. One graph corresponds to the titration of 100 mL 0.10 M acetic acid with 0.10 M NaOH, and the other corresponds to the titration of 100 mL 0.10 M NaOH with 0.10 M acetic acid. Which graph corresponds to which titration? Justify your answer.
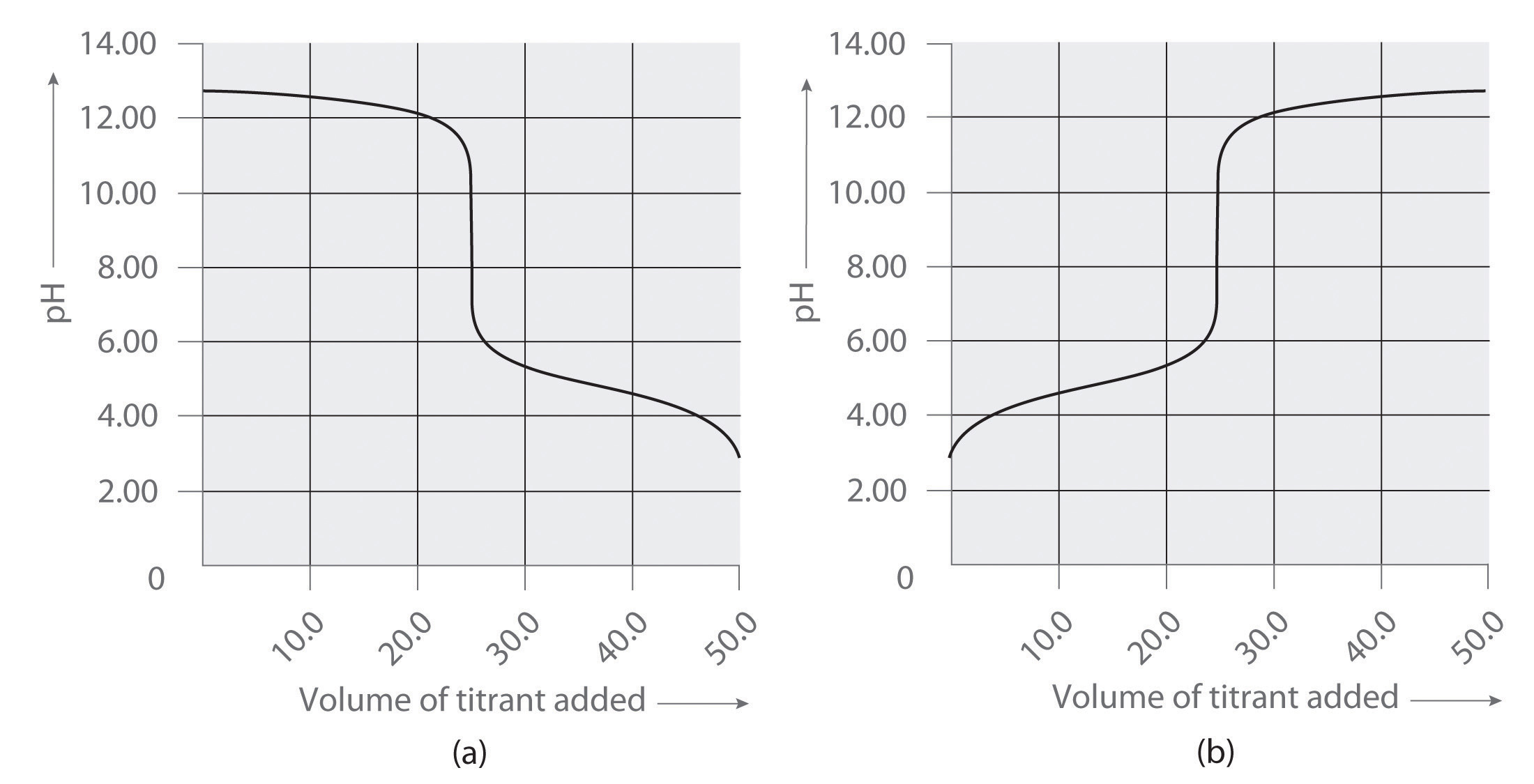
-
Following are two graphs that illustrate how the pH of a solution varies during a titration. One graph corresponds to the titration of 100 mL 0.10 M ammonia with 0.10 M HCl, and the other corresponds to the titration of 100 mL 0.10 M NH4Cl with 0.10 M NaOH. Which graph corresponds to which titration? Justify your answer.

-
Following are two graphs that illustrate how the electrical conductivity of a solution varies during a titration. One graph corresponds to the titration of 100 mL 0.10 M Ba(OH)2 with 0.10 M H2SO4 , and the other corresponds to the titration of 100 mL of 0.10 M NaOH with 0.10 M H2SO4. Which graph corresponds to which titration? Justify your answer.
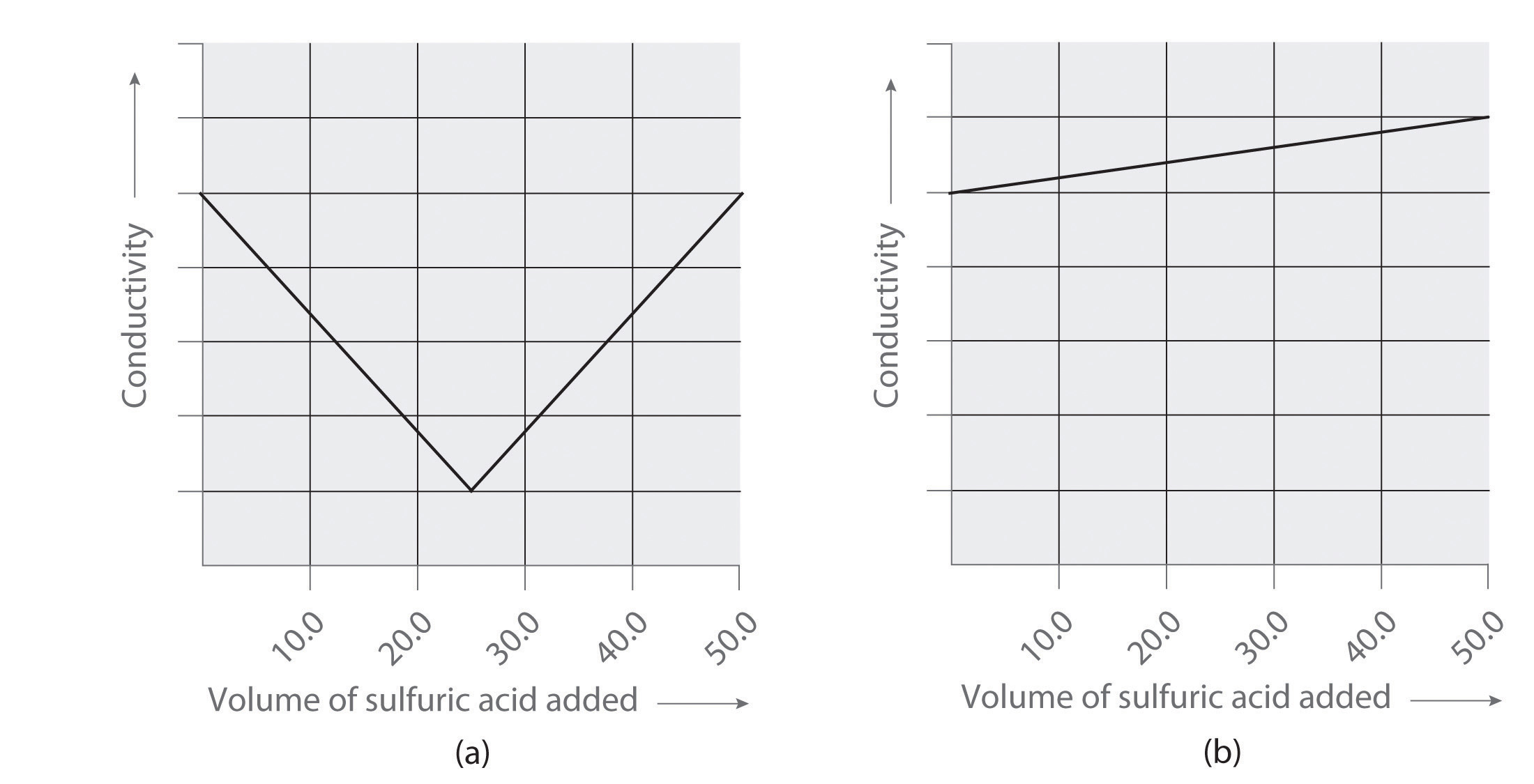
Conceptual Answers
- In a titration procedure, the analyte is considered to be the limiting reactant, as the amount of moles of it remain constant while more and more titrant can be added (making the titrant the excess reactant). By using the fact that the analyte is limited, we can continually add the excess reactant to find out just how many moles are in the analyte.
- To determine the concentration of a solution, one must titrate it with an acid (if the solution is a base) or a base (if the solution is an acid). By titrating the unknown solution with a solution of known concentration, we can determine how many moles of the titrant were needed to fully neutralize the analyte. This point can be determined by finding the equivalence point during the titration, and from this knowledge as well as the mole ratio from the balanced neutralization equation, we can determine how many moles are in the analyte. With this, we can simply divide by the original volume to find its concentration.
-
Graph (a) corresponds to the titration of NaOH with acetic acid, while graph (b) corresponds to the titration of acetic acid with NaOH. This can be determined due to our knowledge that acids have a pH below 7 while bases have a pH above 7. Therefore, titrating a base with an acid would decrease its pH while titrating an acid with a base would increase its pH.
- Graph (a) corresponds to the titration of ammonia with HCl, while graph (b) corresponds to the titration of NH4Cl with NaOH. This can be determined due to our knowledge that acids have a pH below 7 while bases have a pH above 7. Therefore, titrating a base with an acid would decrease its pH while titrating an acid with a base would increase its pH.
-
- titration of Ba(OH)2 with sulfuric acid
- titration of NaOH with sulfuric acid
Numerical Problems
- A 10.00 mL sample of a 1.07 M solution of potassium hydrogen phthalate (KHP, formula mass = 204.22 g/mol) is diluted to 250.0 mL. What is the molarity of the final solution? How many grams of KHP are in the 10.00 mL sample?
- What volume of a 0.978 M solution of NaOH must be added to 25.0 mL of 0.583 M HCl to completely neutralize the acid? How many moles of NaOH are needed for the neutralization?
- A student was titrating 25.00 mL of a basic solution with an HCl solution that was 0.281 M. The student ran out of the HCl solution after having added 32.46 mL, so she borrowed an HCl solution that was labeled as 0.317 M. An additional 11.5 mL of the second solution was needed to complete the titration. What was the concentration of the basic solution?
Numerical Answers
-
Final Molarity = 0.0428M, and there are 0.76g of KHP in the initial sample.
-
15mL of 0.978 M NaOH, or 0.0147 moles.
-
Concentration of basic solution = 0.536 M.

