10.19: Solubility and Molecular Structure
- Page ID
- 49673
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Chemical theory has not reached the point where it can predict exactly how much of one substance will dissolve in another. The best we can do is to indicate in general terms the relationships between solubility and the microscopic structures of solute and solvent.
To begin with, moving particles of any kind tend to become more randomly distributed as time passes. If you put a layer of red marbles in the bottom of a can and cover it with a second layer of white marbles, shaking the can for a short time will produce a nearly random distribution. The same principle applies on the microscopic level. Moving molecules tend to become randomly distributed among one another, unless something holds them back. Thus gases, whose molecules are far apart and exert negligible forces on one another, are all completely miscible with other gases.
In liquid solutions, the molecules are much closer together and the characteristics of different types of molecules are much more important. In particular, if the solute molecules exert large intermolecular forces on each other but do not attract solvent molecules strongly, the solute molecules will tend to group together. This forms a separate phase and leaves the solvent as a second phase. Conversely, if the solvent molecules attract each other strongly but have little affinity for solute molecules, solvent molecules will segregate, and two phases will form.
A classic example of the second situation described in the previous paragraph is the well-known fact that oil and water do not mix—or if they do, they do not stay mixed for long. The reason is that oil consists of alkanes and other nonpolar molecules, while water molecules are polar and can form strong hydrogen bonds with each other. Suppose that alkane or other nonpolar molecules are randomly dispersed among water molecules, as shown in part a of the figure.The constant jostling of both kinds of molecules will soon bring two water molecules together. Dipole forces and hydrogen bonding will tend to hold the water molecules together, but there are only weak London forces between water and nonpolar molecules. Before long, clusters of water molecules like those in part b will have formed.
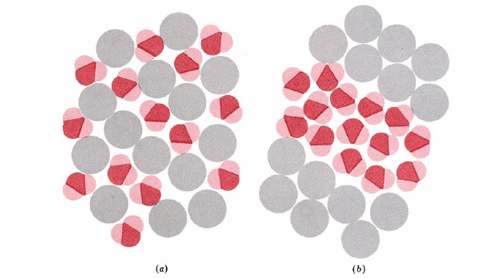
These clusters will be stable at room temperature because the energy of interaction between the water molecules will be larger than the average energy of molecular motion. Only an occasional molecular collision will be energetic enough to bump two water molecules apart, especially if they are hydrogen bonded. Given enough time, this process of aggregation will continue until the polar molecules are all collected together. If the nonpolar substance is a liquid, this process corresponds on the macroscopic level to the liquids separating from each other and forming two layers.
If instead of mixing substances like oil and water, in which there are quite different kinds of intermolecular attractions, we mix two polar substances or two nonpolar substances, there will be a much smaller tendency for one type of molecule to segregate from the other. Thus two alkanes like n-heptane, C7H16, and n-hexane, C6H14, are completely miscible in all proportions. The C7H16 and C6H14 molecules are so similar (recall the projection formulas of alkanes) that there are only negligible differences in intermolecular forces. Thus the molecules remain randomly mixed as they jostle among one another.
For a similar reason, methanol, CH3OH, is completely miscible with water. In this case both molecules are polar and can form hydrogen bonds among themselves, and so there are strong intermolecular attractions within each liquid. However, CH3OH dipoles can align with H2O dipoles, and CH3OH molecules can hydrogen bond to H2O molecules, and so the attractions among unlike molecules in the solution are similar to those among like molecules in each pure liquid. Again there is little tendency for one type of molecule to become segregated from the other.
All the cases just discussed are examples of the general rule that like dissolves like. Two substances whose molecules have very similar structures and consequently similar intermolecular forces will usually be soluble in each other. Two substances whose molecules are quite different will not mix randomly on the microscopic level. In general, polar substances will dissolve other polar substances, while nonpolar materials will dissolve other nonpolar materials. The greater the difference in molecular structure (and hence in intermolecular attractions), the lower the mutual solubility. The following video succinctly showcases this principle.
In the video a number of mixing events occur. A nonpolar, colored solid is added to CCl4. Since CCl4 is also nonpolar, like dissolves like, and the solid is dissolved. Next, water is added. Since water is polar, it does not mix with the CCl4 solution, even after vigorous shaking. Two layers remain, with the less dense water on top. Finally, hexane is added. Since it is nonpolar and less dense than water, it forms a third layer, on top of the water. When the test tube is shaken, however, two layers remain. Since hexane is nonpolar, it is miscible with CCl4, and so both form a single layer below the water.
Predict which of the following compounds will be most soluble in water:
- \(\underset{\text{Ethanol}}{\mathop{\text{CH}_{\text{3}}\text{CH}_{\text{2}}\text{OH}}}\,\)
- \(\underset{\text{Hexanol}}{\mathop{\text{CH}_{\text{3}}\text{CH}_{\text{2}}\text{CH}_{\text{2}}\text{CH}_{\text{2}}\text{CH}_{\text{2}}\text{CH}_{\text{2}}\text{OH}}}\,\)
Solution
Since ethanol contains an OH group, it can hydrogen bond to water. Although the same is true of hexanol, the OH group is found only at one end of a fairly large molecule. The rest of the molecule can be expected to behave much as though it were a nonpolar alkane. This substance should thus be much less soluble than the first. Experimentally we find that ethanol is completely miscible with water, while only 0.6 g hexanol dissolves in 100 g water.
Our discussion of solubility in terms of microscopic structure concludes with one more point. The solubility of other substances in solids are usually small. The constituent particles in a solid crystal lattice are packed tightly together in a very specific geometric arrangement. For one particle to replace another in such a structure is very difficult, unless the particles are almost identical. The most common solid solutions are alloys, in which one essentially spherical metal atom replaces another. Thus alloys are easily made by melting two metals and cooling the liquid solution. In many other cases, however, completely miscible liquids separate when a solid phase forms. A good example of this is benzene and naphthalene:
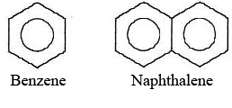
A naphthalene molecule is almost twice as big as a benzene molecule and cannot fit in the benzene lattice. Therefore, even though the liquids are miscible, the solids are not due to the molecular structures of benzene and naphthalene.


