6.12: Alkene Polymerisation- Living Cationic Methods
- Page ID
- 200847
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The trouble with reactive intermediates such as cations is that they are so reactive. Sometimes, cations do unexpected things. For example, we have already seen how they frequently undergo 1,2-hydride shifts. They can undergo nucleophilic addition, but they may undergo elimination instead. Sometimes, there may be competition between different nucleophiles racing to combine with the cation; you may end up with a mixture of products.
In other words, all kinds of things could go wrong during a cationic polymerisation.
What are the consequences of a polymerisation gone bad? The biggest problem is that the polydispersity gets too high. The polymer chains obtained in the reaction are not uniform in size; there are some much, much longer chains as well as some much, much shorter chains.
Remember, the polydispersity is an important component of how we think about the size of polymer chains. Because polymers are a collection of molecules formed through tandem growth of chains -- lots of chains growing at the same time -- we will always have a range of chain lengths. The polydispersity tells us how broad is that distribution. If the polydispersity is too high, it means there is too great a range of chain lengths (and molecular weights) in the material. Because the physical properties of polymers depend in part on the chain length, when the polydispersity gets too high, we have less and less control over the properties of the material. It might not perform the way we want it to perform.
Why do these unexpected reactions of cations lead to a wider range of molecular weights? It's because they often stop a chain from growing. We call these reactions "random termination events".
As a result of random termination events, some chains stop growing when they are still too short. Other chains keep growing and gobble up the rest of the monomer. There is extra monomer left over, because some chains didn't use theirs, so the chains that keep growing get extra long. There is a very wide range of chain lengths.
A secondary symptom of this problem is that the measured molecular weight of the polymer is sometimes higher than expected. That doesn't make any sense at first; if the chains stop growing, how can the molecular weight be higher than we thought it would? Wouldn't it be lower?
Well, sometimes that's true. If all of the chains were to stop growing early, then the molecular weight wouldn't reach our expectations, and there would be a lot of monomer left over. However, we're talking about random termination. Some chains stop growing. Others don't.
Remember that at the end of a polymerization, the polymer is usually precipitated. Any leftover monomers stay dissolved and are decanted off. At the same time, many of the very short chains also stay dissolved, and they are lost, too. We are left with the bigger chains, and the average weight of the chains has shifted higher. In addition, there are physical methods for measuring the size of polymers that are more strongly influenced by the bigger molecules, and that fact may artificially inflate our estimate of molecular weight.
In contrast to this scenario, a "living polymerisation" is a process in which the polymer chains keep growing uniformly and the molecular weight agrees closely with expectations. Furthermore, after a living polymerization has finished, you can add more monomer and the chains will start growing again. The growing chains don't die out and stop growing. When they run out of monomer, they remain "dormant" (it's like they are just sleeping) until more monomer is available.
A key strategy for living polymerisation is to limit the number of reactive species at a given time. The fewer reactive species there are, the fewer random termination events will occur.
That scenario might seem to pose a different problem: if there are fewer reactive species, then there are fewer growing chains. If there are fewer growing chains, won't the polymerisation be much slower? Yes, it will be slower. We are looking for an optimum point at which polymerisation proceeds at a reasonable rate but the termination rate is very low.
However, there is a way to keep the number of reactive species low, but still have lots of growing chains, or at least potentially growing chains. We just exploit an equilibrium in which chains spend part of their time actually growing and part of their time dormant.
The idea is to have an anion that can cap the cation, so that we have an alkyl halide rather than a cation. In a very simple case, we might think about adding some halide salts to the polymerisation reaction. The halide anions might bind reversibly to the cationic intermediate, sending it into a dormant phase. Occasionally, the halide would dissociate again, and the polymer would grow again.
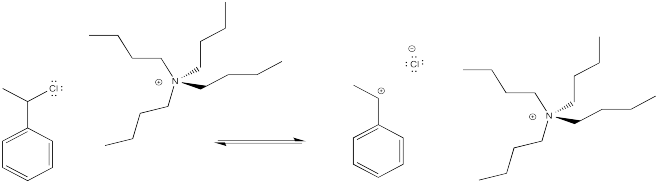
However, the halide must leave once in a while, producing a cation that can undergo polymerisation. In order to help that halide leave, a Lewis acid might be employed. The following example shows tin(IV) chloride added to the mix.
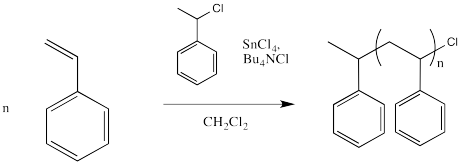
The halide coordinates to the Lewis acid, which polarises the carbon-halogen bond. Tin, iron or titanium compounds are some exampes of Lewis acids that are sometimes used for this purpose, but there are others, as well.
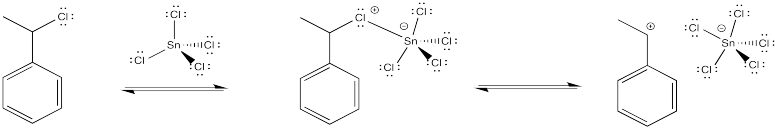
This method may actually change the mechanism of the reaction slightly. Maybe the halide ion never actually leaves completely, and the cation never fully forms. Instead, there may be enough polarization in the presence of the Lewis acid so that the alkene donates to the incipient (almost-formed) cation.
Provide a mechanism, with arrows, for the following living cationic polymerisation reaction.



