6.11: Alkene Polymerisation
- Page ID
- 200846
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We have already seen the general concept that alkenes can be polymerised through a series of electrophilic additions. In this section, we will look at this topic in more depth.
In polymerisation, a large group of monomers are pulled together into a single, large molecule. In most cases, the monomers are connected in a row, forming a long chain. This process is sometimes called "enchainment".
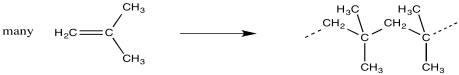
Looking at the structure of the polymer, we can see where the monomers have ended up. They are linked together along the chain in a repetitive fashion. When the monomers become enchained, they turn into the "repeat units" of the polymer.
The structure of a polymer is often depicted using the repeat unit in parentheses. A subscript n stands for an integer, meaning that n of these repeat units are linked together in a chain.
Of course, this chain has to end somewhere. At either end of the chain there will be something else attached; these parts of the polymer are called the "end groups". The identity of these groups can vary; it depends on how the polymer was made. One of these end groups gets incorporated into the polymer when the polymer starts growing; it was the "initiator". The other end group gets incorporated when the polymer stops growing, in a chain termination step. We'll see more about termination soon.

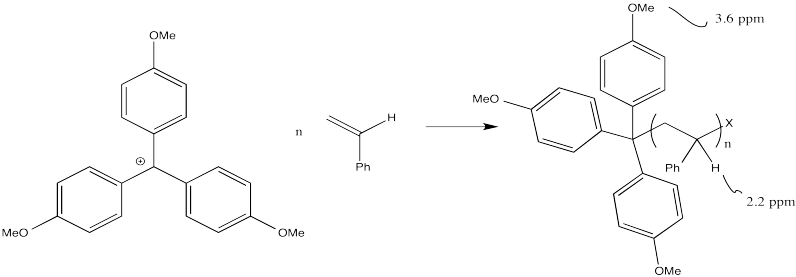
Let's take a look at how a macromolecule grows via cationic polymerisation. The initiator is a cation that reacts with the alkene. When it does so, it forms a new cation from the old alkene.
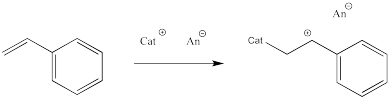
This sort of reaction is called a chain reaction because one reactive species reacted to form a new reactive species; this cycle then keeps repeating in a chain.
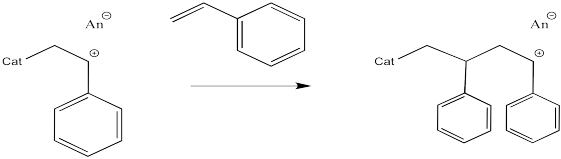
The process keeps repeating so that more and more monomers become enchained. In the picture below, only three monomers have been enchained so far, but you get the idea.
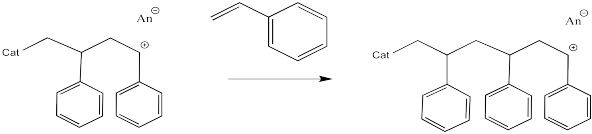
Most cationic polymerisations are initiated by a proton. That means that one of the end groups can be thought of as a proton. Alternatively, the first monomer can be thought of as having a slightly different structure than the repeat units that follow.
Perhaps the most obvious method of providing a proton is to add a protic acid, such as sulfuric acid (H2SO4) or trifluoromethanesulfonic acid (CF3SO3H or often abbreviated as TfOH). However, although that seems easy on paper, polymerisations typically don't work extremely well under those conditions. Usually, something goes wrong and the polymer stops growing when it is still a fairly short chain.
More commonly, commercial polymerisation involves a two-component mixture that together can initiate the reaction. One of these components is a Lewis acid. Some common Lewis acids used for this purpose include BF3, AlCl3, TiCl4 or SnCl4. The other component is usually a small amount of water or alcohol.
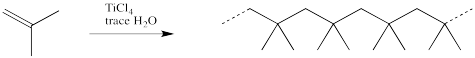
The role of the Lewis acid is to activate the water or alcohol, providing a proton to initiate a growing chain. Because the water or alcohol is the actual source of the proton, it is referred to as the initiator, whereas the Lewis acid is called a co-initiator.
The other end group in the polymer is formed during termination, when something happens that stops the polymer from going. In termination, the cation in the growing chain would be lost. That event can happen through a few different pathways.
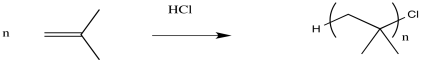
The simplest thing that might happen to stop chain growth is for some anion to connect irreversibly with the cation of the growing chain. That might happen accidentally while the polymerisation is supposed to be occurring. Alternatively, it might be forced to happen on purpose. When the polymer has grown for a long enough time to reach the desired weight, we might simply add some aqueous acid (like dilute HCl, for example). In that case, chloride ions might connect with the cations. Also, water molecules might connect with the cations, leaving the chains terminated by hydroxyl groups after loss of a proton.

Other accidental pathways for chain termination include "chain transfer" events. In chain transfer, a proton is lost, either to an anion or to an alkene. This event results in an elimination reaction, forming an alkene at the end of the chain. However, the transferred proton results in initiation of a new growing chain. These accidental terminations can be a problem because some chains just get started growing after others have already been growing for a long time. As a result, the material obtained has a variety of chain lengths. The material has a high polydispersity, which means the properties of the material are difficult to control.

Termination events also raise the possibility that there will be some leftover monomer at the end of polymerisation. We wouldn't want these small molecules in the material we are making, because they would slowly leach out of the material over time. Sometimes, these monomers are removed via a precipitation step. The polymer mixture is simply dumped into a solvent in which the monomers will dissolve but the polymer will not. The solvent is then poured off, taking the monomers with it, leaving behind a purified polymer.
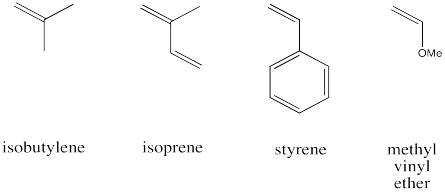
The following monomers are readily polymerised via cationic polymerisation. Show why, using drawings of the intermediates invlved in the reaction in each case.