4.18: Nucleophilic Substitution at Silicon
- Page ID
- 200814
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Silicon is in the same group as carbon in the periodic table, so in some ways it might be expected to behave in some ways that are similar to carbon. It might not be surprising that silicon can undergo nucleophilic substitution reactions like carbon. However, there are differences in both the mechanism and the reactions that are likely to occur.
Probably the most common example of nucleophilic substitution at silicon occurs during the formation of silicone polymers. Silicon forms a pretty strong bond to oxygen, so a chloride leaving group is easily displaced from silicon to form a silanol. If a dichlorosilane is exposed to water, a silane diol is produced.
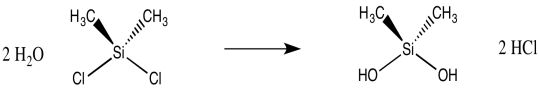
Just by adjusting the ratio of silicon that is reacting with water, we could imagine forming different products. A 2:1 ratio of H2O : Me2SiCl2 might lead to a silane diol, Me2Si(OH)2. However, a 3:2 ratio could end up forming a silicone dimer, Me2(HO)SiOSiMe2(OH). That's because the hydroxy group on the first silicon atom is still a nucleophile, and it could donate to a second silicon atom, forming a bridge between two silicon atoms.
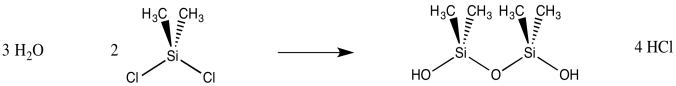
If the water is introduced gradually to the dichlorosilane, polymerization results, because a chlorosilane is more likely to react with a neighbouring silanol than with scarce water molecules. Lots of Si-O-Si bridges form, leading to a polymeric material: silicone rubber.
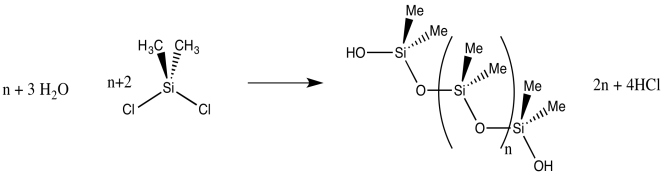
- Silicones are polymers containing -(R2SiO)- repeating units.
- Silicones are formed via polymerization of dichlorosilane compounds with water.
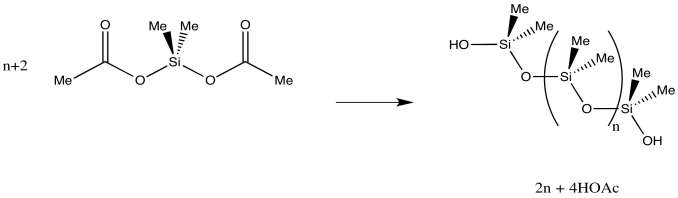
The side product of this reaction is hydrogen chloride, HCl. When it contacts water, HCl dissociates to give hydrochloric acid. Hydrochloric acid is a strong acid and a severe health risk. Because of that, there are alternative formulations for making silicone that are a little safer. If the chlorine is replaced with an acetate group, polymerization is still possible upon exposure to water. However, the side product is the somewhat safer acetic acid. This route is used in commercially available silicones for household use. For example, silicone caulk can be used to waterproof around a bathtub or sink. The strong smell of vinegar when it is left to cure is the acetic acid being produced when the acetoylsilane reacts with moisture in the air.
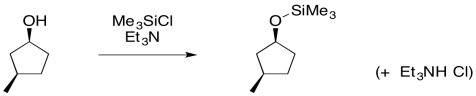
Apart from its use in polymer materials, substitution at silicon plays an important role in organic synthesis. Silyl ethers can be made in ways similar to the formation of regular ethers. The usual approach is through addition of an alcohol to a chlorosilane. The presence of a weak base, such as a tertiary amine, prevents the buildup of corrosive hydrochloric acid. That's what would result if the chloride leaving group were the only base available to pick up the extra proton.
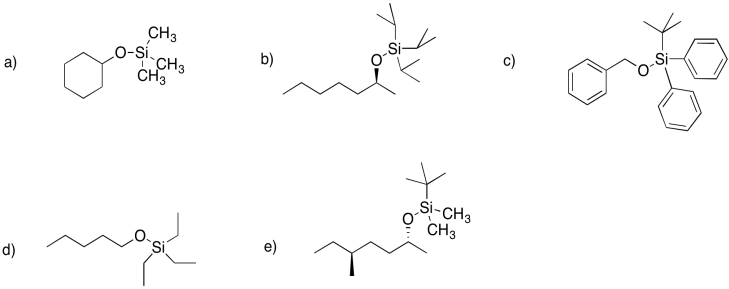
Draw structures for the following silyl ethers.
a) the trimethylsilyl ether of cyclohexanol
b) the triisoproylsilyl ether of (S)-2-heptanol
c) the tert-butyldiphenylsilyl
d) the triethylsilyl ether of pentanol
e) the tert-butyldimethylsilyl ether of (S)-5-methylheptan2-ol
- Answer
-
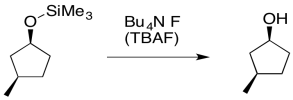
The chief utility of silyl ethers is that they are just as easy to break down as they are to make in the first place. They can be converted back to alcohols in the presence of hydrochloric acid, although the process can be very slow. That's one of the reasons that HCl must be removed during siliyl ether formation. There is an equilibrium at work here, and at some point a buildup of HCl would drive the reaction backward, preventing further formation of the silyl ether.
Silyl ethers can be cleaved much more quickly in the presence of fluoride ion. The usual reagent for this transformation is tetrabutylammonium fluoride (TBAF). The tetrabutylammonium ion is pretty soluble in most organic solvents; that makes the reagent much easier to use.
The reason that reversibility is useful is because it offers a way to temporarily "cover up" and alcohol group. Alcohols can be problematic during many reactions. The slightly acidic OH group, like the slightly acidic OH group of a water molecule, can interfere with sensitive reagents. Compounds that are highly basic, such as alkylmetal compounds (e.g. BuLi, EtMgBr, etc) and metal amides (e.g. NaNH2, LDA, etc) will deprotonate that OH group instead og going on with their intended business with the reactant. For example, if a Grignard reagent is supposed to add to a carbonyl, but it encounters a hydroxy group, it will simply pull the proton from the hydroxy group. Then it will be neutralised. It won't be a nucleophile anymore, so nothing will happen after that.
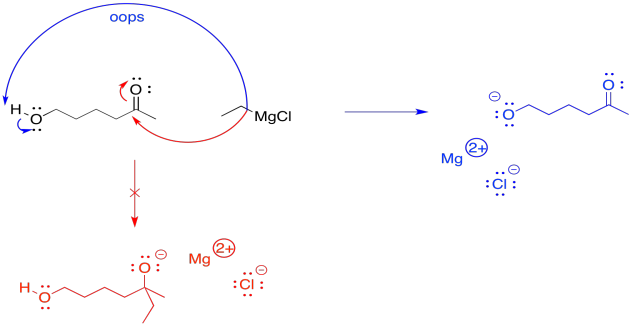
Instead, if the hydroxy group is first protected, the reaction will proceed with no possibility of an accidental acid-base event. Later, the hydroxy group can easily be replaced.
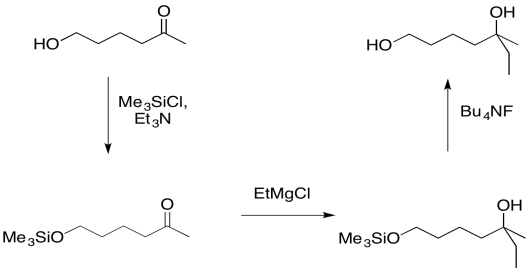
- Silyl ethers can be used to protect mildly acidic alcohols
- Silyl ethers can be removed easily when protection is no longer needed
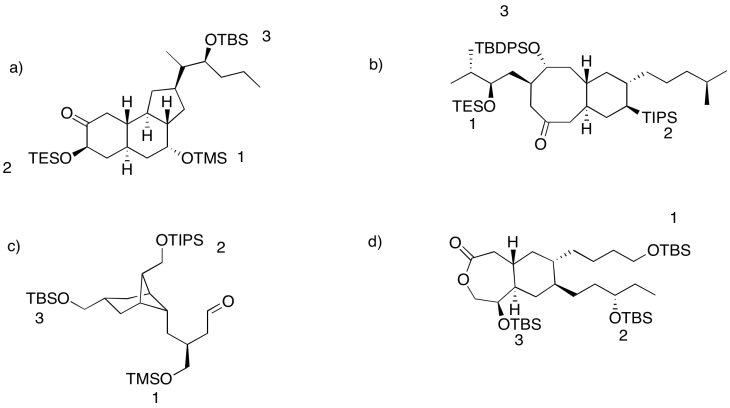
Common silyl ethers include trimethyl silyl (Me3Si, or TMS); triethylsilyl (Et3Si, or TES); tri-iso-propylsilyl (iPr3Si, or TIPS); tert-butyldimethylsilyl (tBuMe2Si, or TBS); and tert-butyldiphenylsilyl (tBuPh2Si, TBDPS). The fact that a variety of silyl ethers are commonly available allows chemists to choose from different ones. As a result, several different silyl ethers might be used to protect alcohols in different positions in a more complicated molecule. They can then choose which silyl group to remove, in which order. Selective removal of silyl ethers is possible because they are very sensitive to steric effects. The more crowded the silyl ether, the harder it is to remove.
- Less crowded silyl ethers are removed most easily
- More crowded silyl ethers are removed more slowly
- Less crowed silyl ethers can be removed, leaving more crowded ones intact
In each of the following cases, rank the order in which the silyl ethers could be removed.
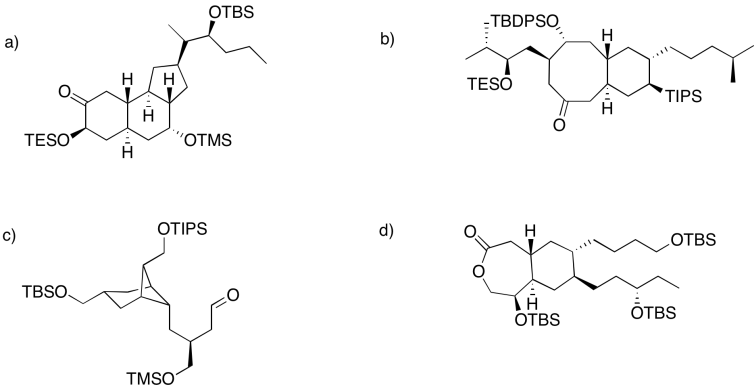
Answer-
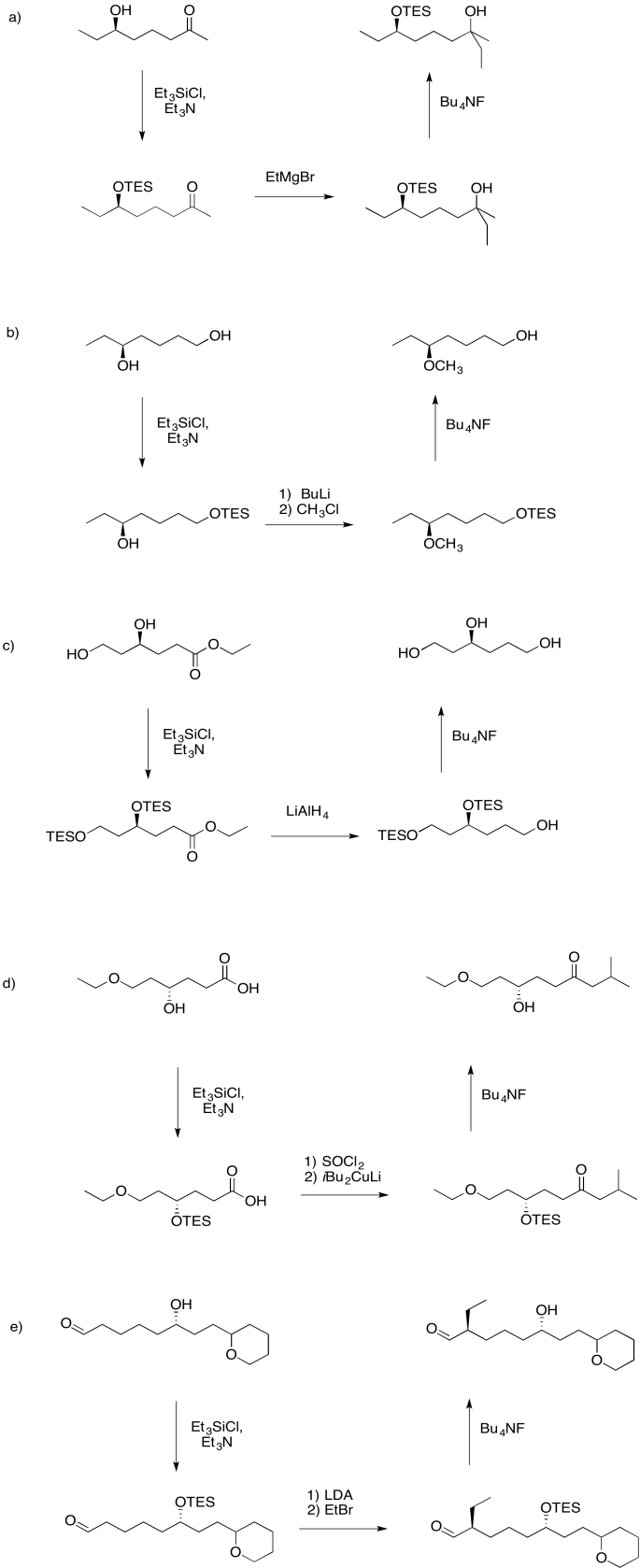
Use a triethylsilyl ether to help complete the following transformations.
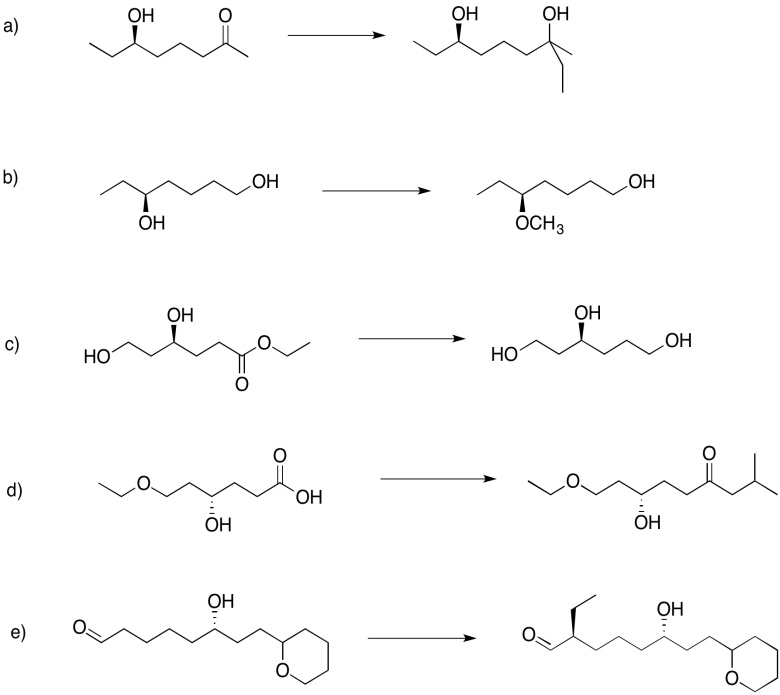
Answer-