3.28: What is a Nucleophile?
- Page ID
- 195732
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Lots of things can be nucleophiles. In principle, a nucleophile only needs a lone pair. However, some nucleophiles are better than others.
You already know something about nucleophiles if you know something about acidity and basicity. Nucleophiles are really Lewis bases. Some of the factors that account for basicity also account for nucleophilicity.
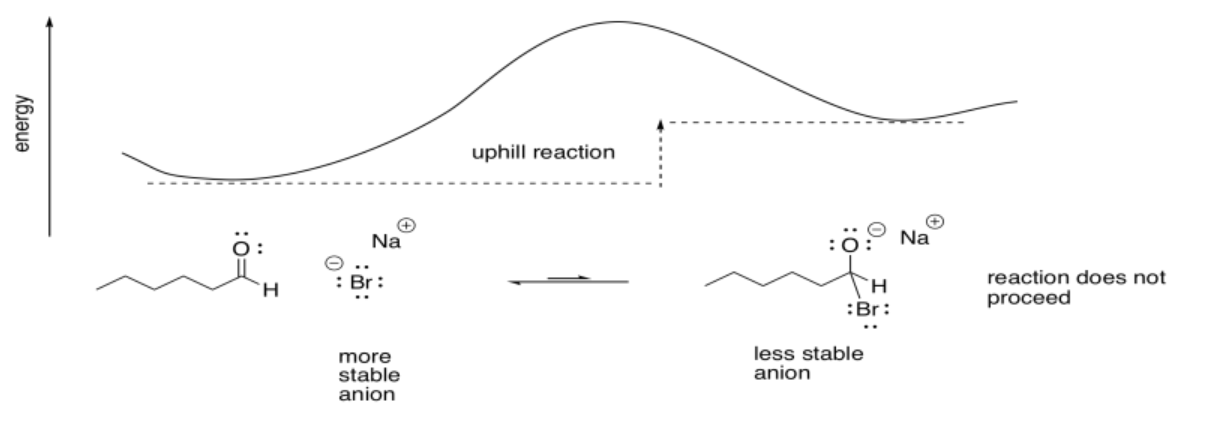
Halides are not very good nucleophiles for carbonyls. The negative charge on a halide is pretty stable, either because of electronegativity or polarizability. If a halide donates to a carbonyl, producing an oxygen anion, the reaction is uphill.
Hydroxide and alkoxide anions (such as CH3O-) are more reactive than halides. They are better nucleophiles. The sulfur analogues are similarly good nucleophiles (such as CH3S-). In addition, water, alcohols and thiols are nucleophilic, because they all have lone pairs that could be donated to an electrophile.
Nitrogen also has a lone pair in most compounds. That means amines are good nucleophiles, too.
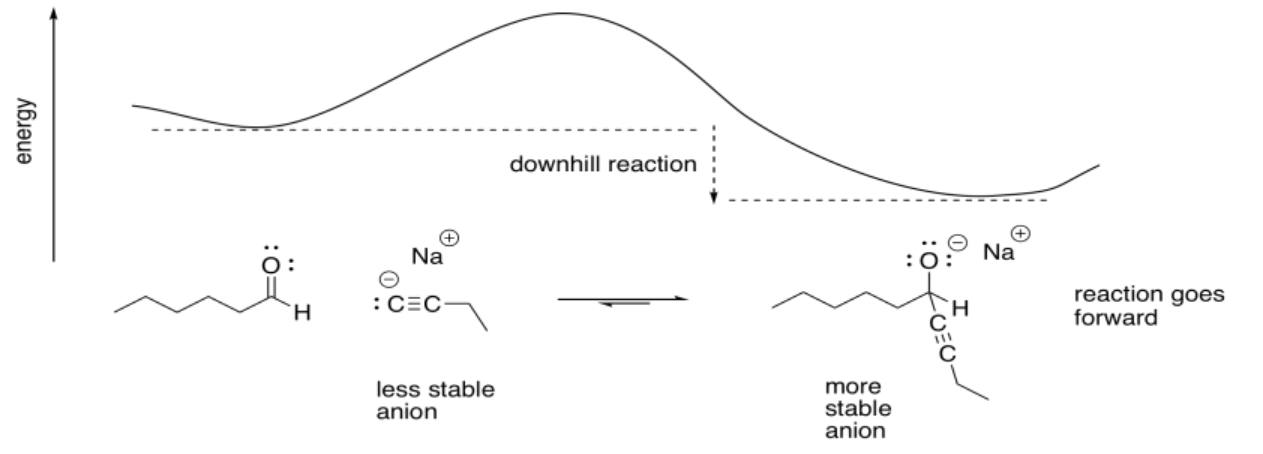
Carbon does not normally have a lone pair, unless it is a carbanion. Carbanions are usually not very stable. As a result, they are not very common, except for cyanide (CN-) and acetylides (RCC-, in which R is a hydrogen or an alkyl group). However, when carbon does have a lone pair (and a negative charge), it is a good nucleophile. Because carbon is less electronegative than other elements with lone pairs, it is able to donate its lone pair easily.
Carbon nucleophiles add to carbonyls because that less stable carbon anion is traded for a more stable alkoxide anion. The reaction is downhill energetically.
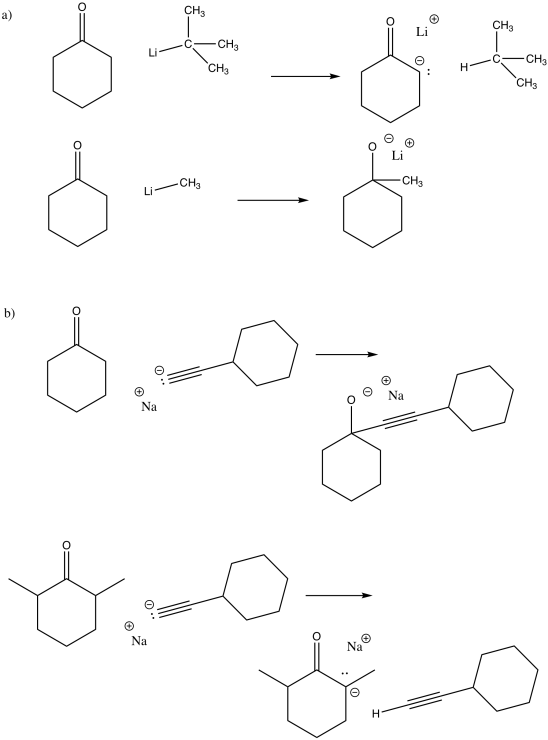
Exercise \(\PageIndex{1}\)
Carbanions such as CH3- (methyl anion) are very unstable and highly reactive. Explain why the following anions are more stable than a methyl anion.
- Acetylide, HCC-
- cyanide, CN-
- Answer a
-
In acetylide, the lone pair is on a linear carbon or sp carbon. In methyl anion, the lone pair is on a tetrahedral carbon or sp3 carbon. The description "sp" indicates that sigma bonding to neighbors involves a 2s orbital and a 2p orbital on carbon; there is a 50% contribution from the s orbital.
The description "sp3", on the other hand, indicates that sigma bonding to neighbors involves a 2s orbital and three 2p orbitals; there is a 25% contribution from the s orbital.
The 2s orbital is lower in energy than the 2p orbital. The greater the s orbital contribution to the bond (or in this case to the lone pair), the lower it is in energy. Thus, a lone pair on an sp carbon is lower in energy than a lone pair on an sp3 carbon.
- Answer b
-
In cyanide, the same argument outlined in part (a) hold true. In addition, the nearby electronegative nitrogen stabilizes the charge by drawing electron density toward itself.
Other nucleophiles, such as halides, do not proceed. They are going uphill, from a more stable halide ion to a less stable alkoxide ion.
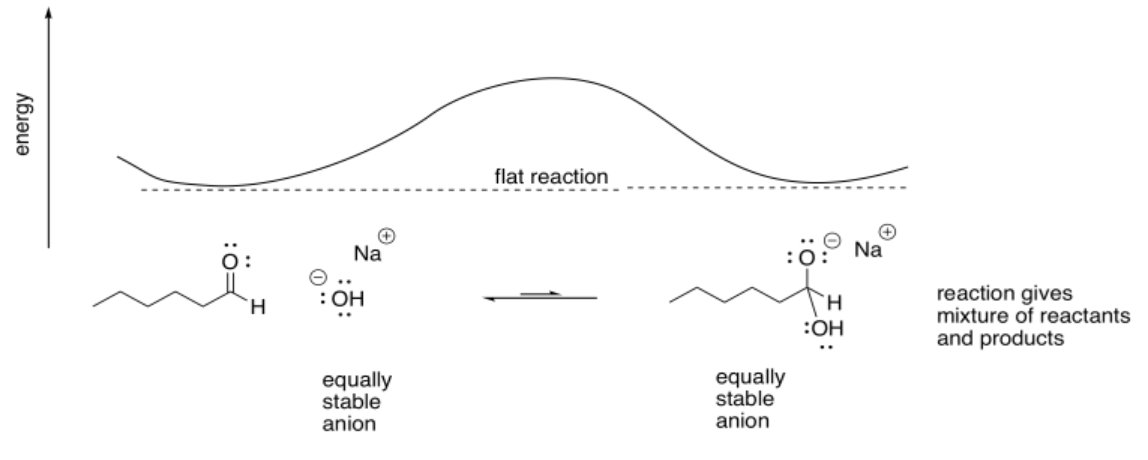
If the nucleophilic atom were an oxygen anion, there might be an equilibrium. The reaction would be neither uphill nor downhill. It would result in a mixture of the original reactants and the new products.
Exercise \(\PageIndex{2}\)
Nucleophilicity is the degree of attraction of a nucleophile to a positive charge (or partial positive charge). It is related to basicity. Choose the most nucleophilic item from each of the following pairs, and explain your answer.
- CH3OK or CH3OH
- CH3OH or CH3NH2
- NaCN or NaCCH
- c-C6H11ONa or c-C6H5ONa (c- in this case means "cyclo")
- Answer a
-
CH3OK, because of the ionic O-K bond. This is an anionic nucleophile. It is more reactive and nucleophilic than the corresponding neutral nucleophile.
- Answer b
-
CH3NH2, because nitrogen is less electronegative than oxygen. Its lone pair is held less tightly and is more easily donated to the electrophile.
- Answer c
-
NaCCH, because the neighbouring carbon in this case does not have the inductive electron-withdrawing effect that the nitrogen does in the case of NaCN. In that case, the lone pair is stabilized and made less reactive.
- Answer d
-
c-C6H11ONa, because the negative charge is localized on one atom. In c-C6H5ONa, the negative charge is delocalized over four different positions in the molecule. Delocalization of charge stabilizes the anion and makes it less reactive.
Exercise \(\PageIndex{3}\)
Carbonyl compounds such as aldehydes and ketones contain a very slightly acidic hydrogen next to the carbonyl. Some nucleophiles are basic enough to remove that proton instead of donating to the carbonyl. Show why the resulting anion is stable, using cyclopentanone as an example.
- Answer
-
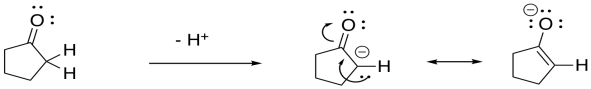
The resulting anion is stabilised by resonance.
Exercise \(\PageIndex{4}\)
Accidental deprotonation (proton removal) alpha to a carbonyl (one carbon away from the carbonyl) can occur when a nucleophile is added to a ketone. One reason the proton might be taken instead is if the carbonyl is too crowded for the nucleophile to reach.
In the following cases, explain which nucleophile is more likely to add to the carbonyl in cyclohexanone and which is more likely to deprotonate it.
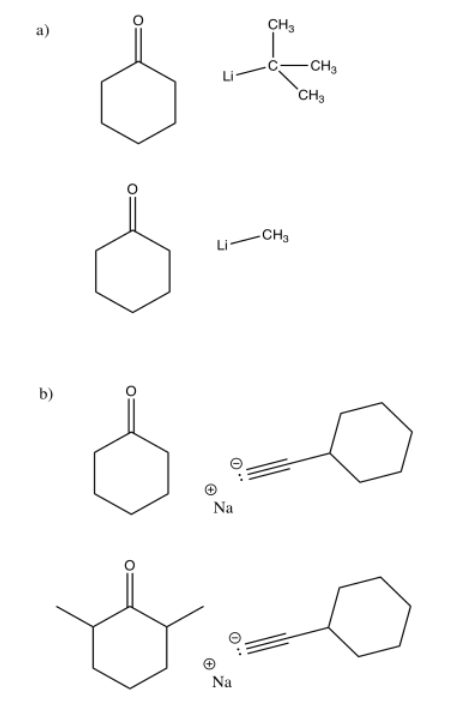
- Answer
-
The case with more steric crowding is more likely to result in deprotonation.