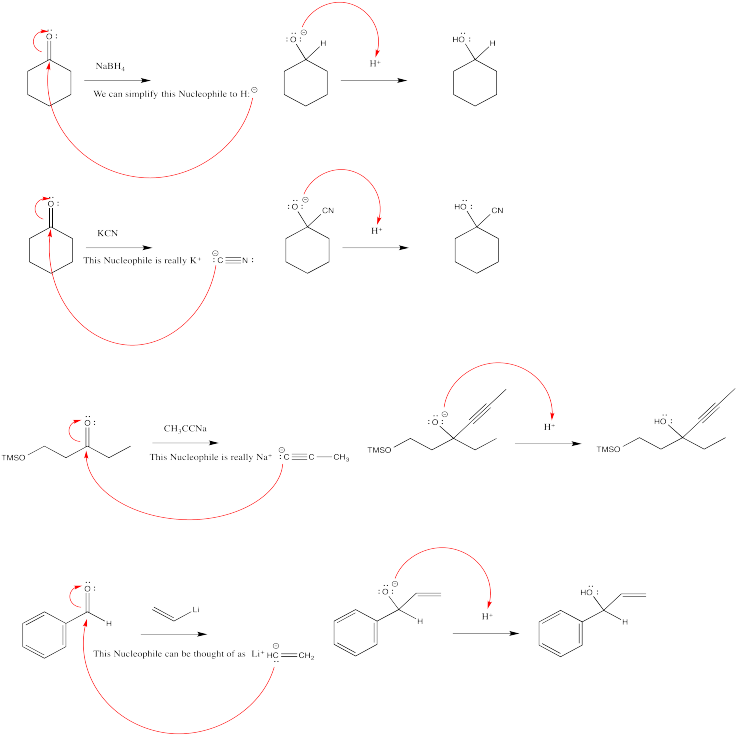
3.27: Protonation of the Alkoxide Anion
- Page ID
- 195731
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After the addition of an anionic nucleophile, reaction mixtures are usually treated with or water or dilute aqueous acid. The acid provides a proton that can be picked up by the alkoxide ion formed in the nucleophilic addition. An alcohol is formed as a result. Overall, carbonyl addition reactions usually involve addition of a nucleophile to the carbonyl carbon and addition of a proton to the carbonyl oxygen.
The order of these steps can be very important. On paper, a carbonyl could be turned into an alcohol by adding a proton to the carbonyl oxygen, and then adding a nucleophile to the carbonyl carbon. However, things might not work out that way in reality. The potential problem lies in the fact that anionic nucleophiles can be pretty basic. If protons are added first, the anionic nucleophile is likely to pick up a proton rather than donate to the carbonyl. Once the nucleophile has picked up a proton, it is no longer anionic, and is less attracted to the partially positive carbonyl. Furthermore, it may have donated its only lone pair to the proton, leaving it completely unable to donate to the carbonyl.
What is the source of protons? In most cases, a dilute aqueous solution of a strong acid is used. That is, a little bit of strong acid is dissolved in water to provide plenty of protons to form the alcohol. The most common strong acids are hydrochloric acid, HCl, and sulfuric acid, H2SO4.

Actually, just the water itself would be enough to donate protons. Water has a polar O-H bond and so it can transfer a positive hydrogen ion to an anion. However, that would result in the exchange of one oxygen anion for another. Energetically, there wouldn't be much difference between having the negative charge on the reaction product, the alkoxide, or on the hydroxide ion from the water. That's why a strong acid is usually added.
In terms of le Chatelier, if you added enough water, you should be able to drive that equilibrium to the right. However, there would always be a little unprotonated alkoxide left over at the end.
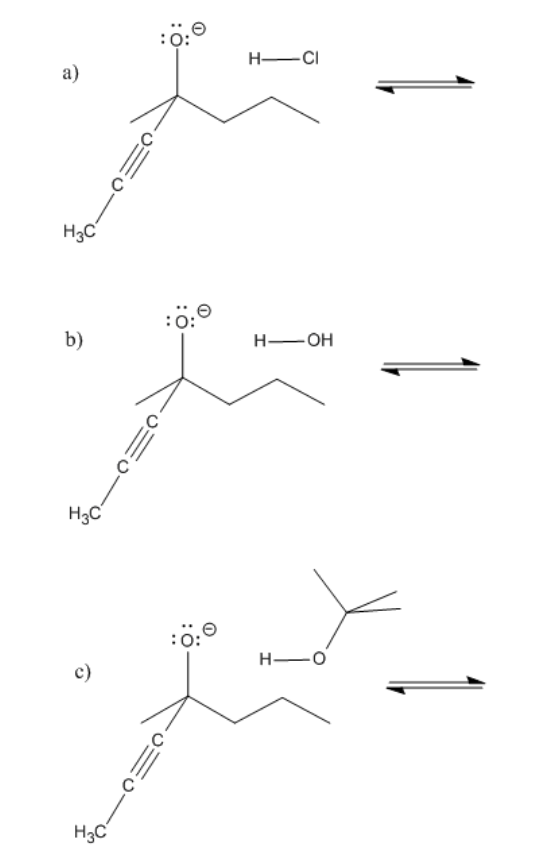
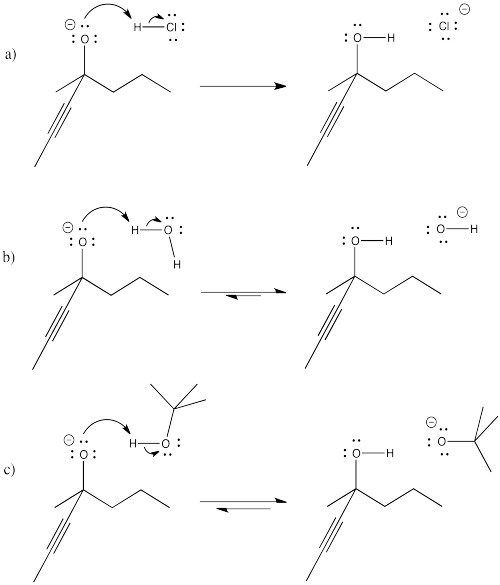
Exercise \(\PageIndex{1}\)
Problem CO8.1.
Provide curved arrows and predict the direction of equilibrium in the following cases.
- Answer
-
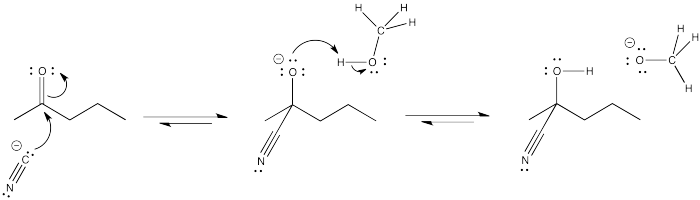
Exercise \(\PageIndex{2}\)
Problem CO8.2.
Suppose nucleophilic addition was performed in methanol (10 mL) as a solvent, using 25 microliters of cyclopentanone and an equimolar amount as the of sodium cyanide (that means the same number of moles of sodium cyanide as cyclopentanone).
- Show the mechanism for the reaction, using curved arrows in each step.
- Comment on how the use of methanol as a solvent (rather than just adding an equimolar amount of methanol) would influence the direction of equilibrium for the final protonation step.
- Answer
-
Exercise \(\PageIndex{3}\)
Provide structures for the following acids. They are moderately strong acids, but not as commonly used in this context as hydrochloric and sulfuric acid. Note that, like sulfuric acid, they all contain polar OH groups.
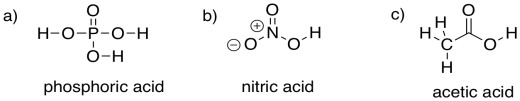
a) phosphoric acid, H3PO4 b) nitric acid, HNO3 c) acetic acid, CH3CO2H
- Answer
-
Sometimes, weak acids are used to protonate the alkoxide intermediate. These acids are more acidic than water, so the equilibrium in the protonation reaction lies more towards the alcohol side. However, because they are only weak acids, they are safer and easier to work with than strong acids. Commone examples include ammonium chloride and sodium bicarbonate.
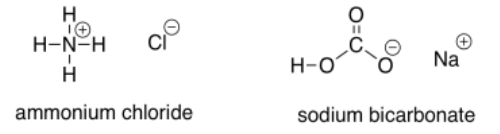
Exercise \(\PageIndex{4}\)
What factor makes each of these compounds mildly acidic?
- Answer
-
Ammonium chloride has an N-H bond, normally less polar than the O-H bond of water. However, the positive charge makes this compound give up a proton more easily, because it results in a neutral (uncharged) ammonia molecule.
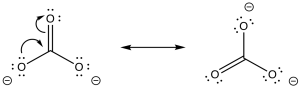
Sodium carbonate has a polar O-H bond, just like water. However, the anion that results from loss of a proton is resonance-stabilised. That makes this compound more acidic than water.
Exercise \(\PageIndex{5}\)
Problem CO8.5.
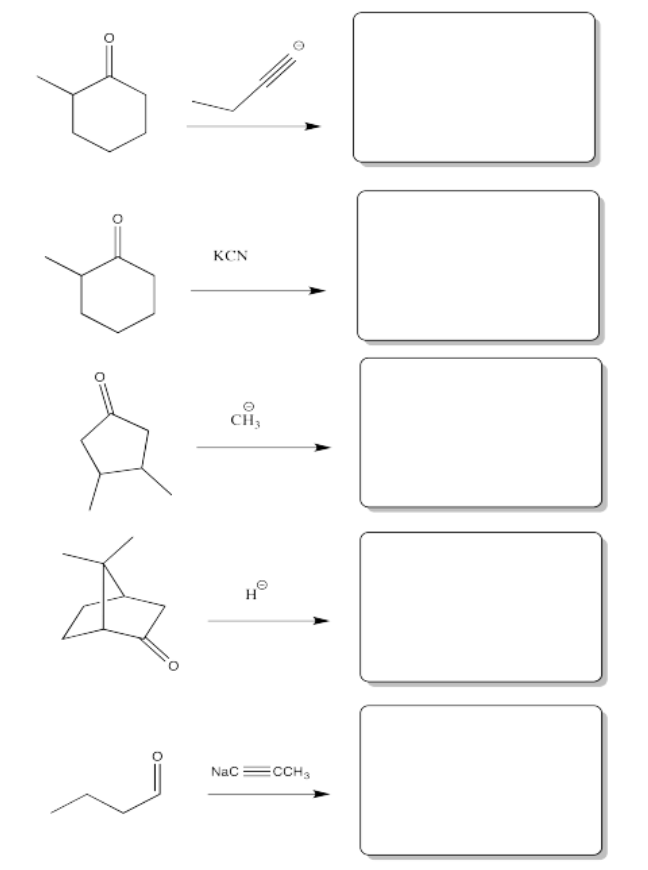
Fill in the product or reagent for each of the following transformations. Remember there is always an acidic workup assumed.
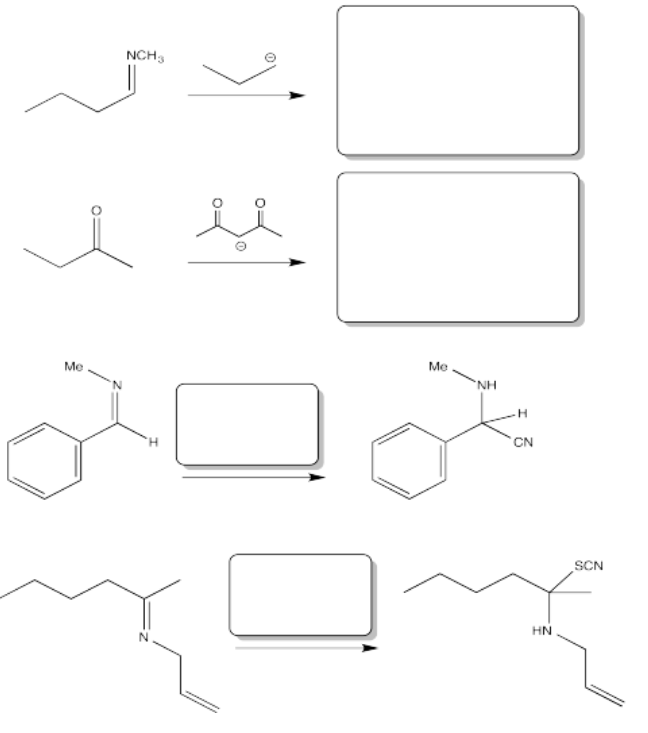
- Answer
-
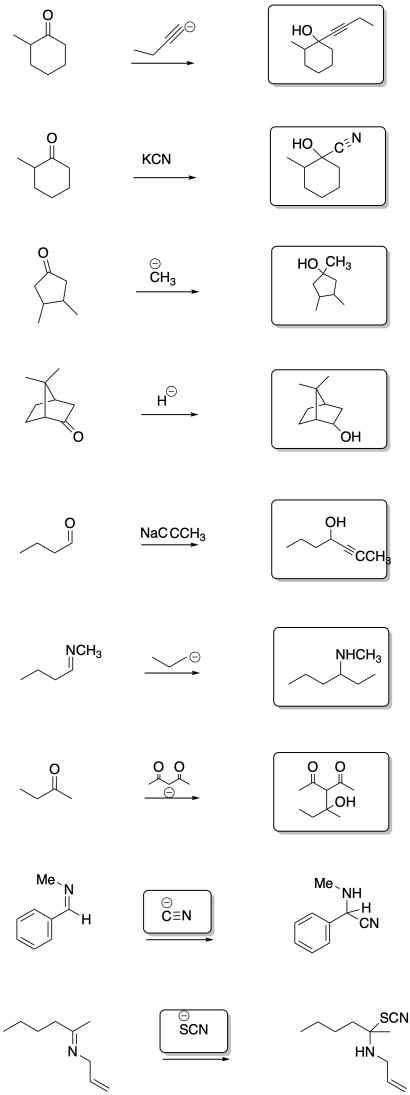
Exercise \(\PageIndex{6}\)
Draw a mechanism with curved arrows for each of the transformations listed below. Show all intermediates. Assume an acidic workup.
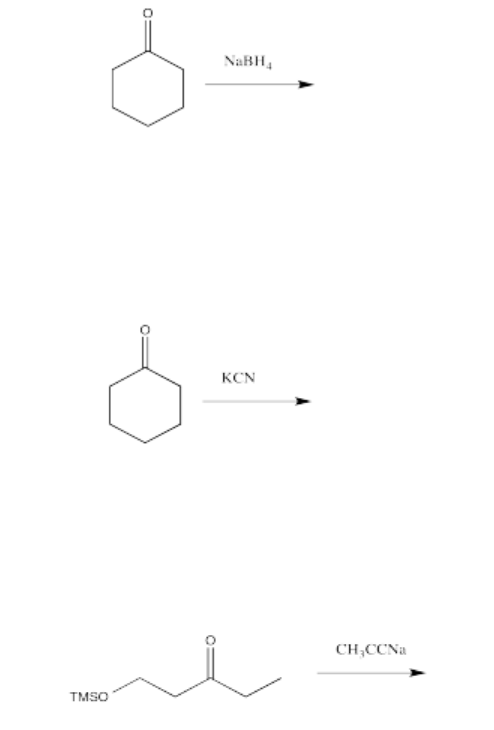
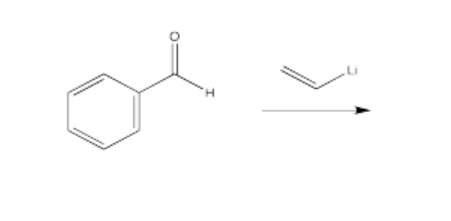
- Answer
-