3.22: General Reactivity Patterns
- Page ID
- 189934
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In the following pictures, a number of anions are added to a simple carbonyl compound, a ketone (2-propanone, or acetone). In each case, addition of the nucleophile is followed by addition of a proton source. Note that, overall, the reaction involves addition of the nucleophile to the carbonyl carbon and addition of the proton to the carbonyl oxygen.
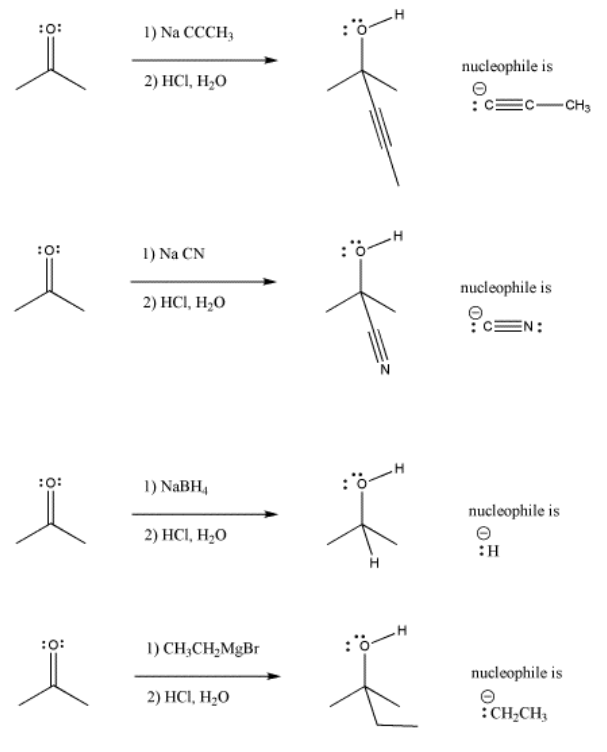
- Addition of anionic nucleophiles to ketones or aldehydes transforms the carbonyl into an alcohol.
Look at the way the reaction is presented in each case. The organic (carbon-based) starting material is presented on the left hand side of the reaction arrow. The reagent added to this starting material is often shown over the arrow. This reagent transforms the starting material into something else. That something else, the product, is shown to the right of the arrow.
Very often, the solvent for the reaction is shown underneath the arrow. The solvent is the liquid that is used to dissolve the starting material and reagents. This is done for a number of reasons. First, reactions generally happen much more quickly in solution than they do without a solvent. When dissolved, the reactants can move around more easily and bump into each other, as if they are swimming. Also, most useful reactions generate heat, and the solvent acts as a heat sink, carrying the excess heat away. (People who have not thought about the importance of solvent sometimes accidentally start fires as a result.) However, there are exceptions, and not all reactions need solvent.
These reactions shown above do need solvent, but the solvent is not shown for other reasons. There is something else to focus on, and the solvent would have just cluttered up the picture. Instead, the focus of the picture is that these reagents must be added in a particular order: first the nucleophile and then the acid. The nucleophile and acid cannot be allowed to mix before the nucleophile has a chance to react with the carbonyl. If they did, they would just react with each other, and leave the carbonyl electrophile alone.
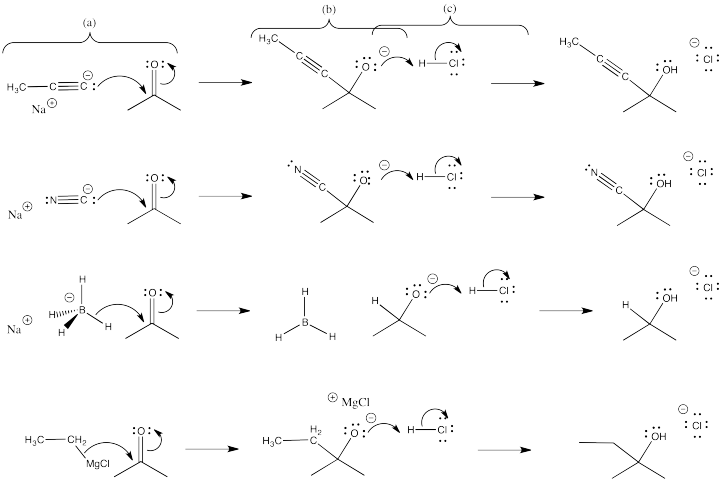
Exercise \(\PageIndex{1}\)
- For each of the cases shown above, use curved arrows to show the movement of electrons in the reaction between the anion and the carbonyl.
- Show the intermediate that results.
- Use curved arrows to show the movement of electrons in the reaction of the intermediate with acid to form the product.
- Answer
-
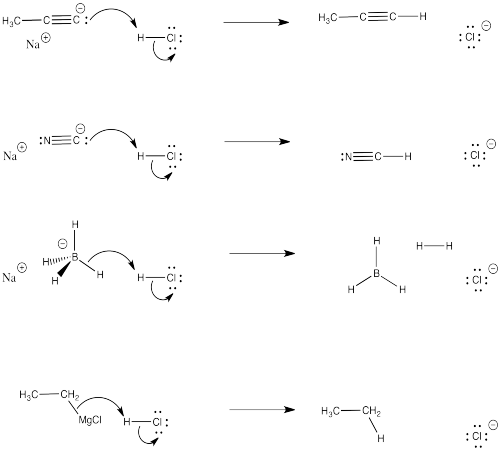
Exercise \(\PageIndex{2}\)
Problem CO3.2.
a) For each of the cases shown above, used curved arrows to show what would happen if acid were mixed with the nucleophile.
b) Why would the nucleophile no longer be able to react with the carbonyl?
- Answer
-
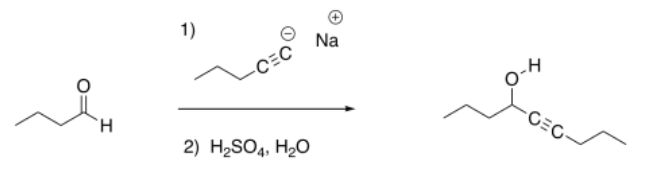
Previously, we saw that nucleophiles add to carbonyl electrophiles, breaking the pi bond of the carbonyl and converting it into an OH group.
The pattern of reactivity is very different with another class of nucleophile. These could be called neutral nucleophiles (as opposed to anionic ones). Neutral nucleophiles do not have a negative charge like the previous ones. However, they still have a lone pair, and that fact still makes them nucleophiles.
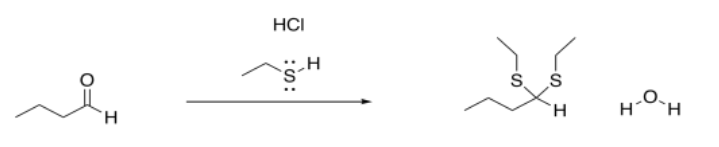
However, the outcome of the reaction looks a little different from what we saw with the earlier anionic nucleophiles. In this case, the carbonyl is not converted into an OH group. (At some point we will see that it can be under some circumstances, but that is the exception rather than the rule.) Instead, the osygen is completely displaced from the carbonyl. It is lost as water. It is replaced by two nucleophiles, instead.
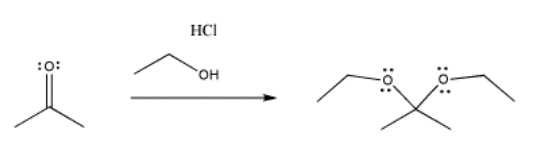
The same thing happens in the following case, involving an oxygen nucleophile instead of a sulfur. Oxygen is in the same column of the periodic table as sulfur, so similar behavior is not really a surprise.
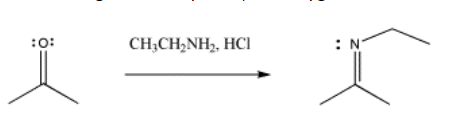
With nitrogen nucleophiles, the oxygen of the carbonyl is still displaced as water, but the nucleophile does not appear to add twice. Instead, the C=O is replaced with a C=N.
Other nitrogen compounds give a similar product, with the double bond in a slightly different place. We will see that the difference has to do with how many hydrogens there are on the original nitrogen atom. If there are two, it is an even trade: the nitrogen replaces the carbonyl oxygen, and the oxygen takes the two hydrogens to form water. If there is only one NH, the oxygen needs to pick up a second hydrogen from elsewhere, and it takes it from the alpha position, next to the former carbonyl.
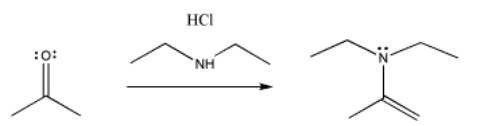
These nucleophiles are a little less likely to react with the acid, so we have not taken care to add them in a particular order. They are less likely to react with the acid because they are not anions. They are neutral, so they will not attract the proton as strongly as anions would. In reality, you have to be a little bit careful about conducting reactions like these. With the amine nucleophiles in particular, if you add too much acid, the nucleophiles will react with the acid after all.
Take another look at the general pattern of reactivity for the anionic nucleophiles and the neutral nucleophiles. In the case of the anionic nucleophiles, the pattern is relatively easy to discover. The product has incorporated the nucleophile into its structure (or at least the anionic part of the nucleophile, which you will soon learn about). The nucleophile has attached at the carbonyl carbon. The carbonyl oxygen has become part of a hydroxyl group. These are very common patterns in the addition of nucleophiles to carbonyls.
In the case of the neutral nucleophiles, there are some similarities and some differences. The nucleophile is still incorporated into the product structure. It has added at the carbonyl position in the electrophile. However, the fate of the carbonyl oxygen is a little bit different with neutral nucleophiles. Generally, this atom is lost as a water molecule in these cases. If you look closely, you will be able to tell where the two protons come from in each case in order to form the water molecule. It's not really the HCl, which is only added in very tiny amounts and acts catalytically. The protons come from other positions in the nucleophile, and sometimes from the electrophile, too.
This chapter will help you to develop skills so that you can recognize where nucleophilic additions have taken place in reactions. You will also be able to predict what products may result from a nucleophilic addition.


