6.11: Other Rings
- Page ID
- 191301
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We have looked at some different six-membered rings, cyclohexanes, and have seen how substituents attached to these rings might influence the shape of the overall molecule. Cyclohexanes are particularly important because of they are quite common in nature. There are other variations of cyclic systems that are worth looking at, including different cyclohexane derivatives and other rings.
Bicyclics and Other Polycyclics
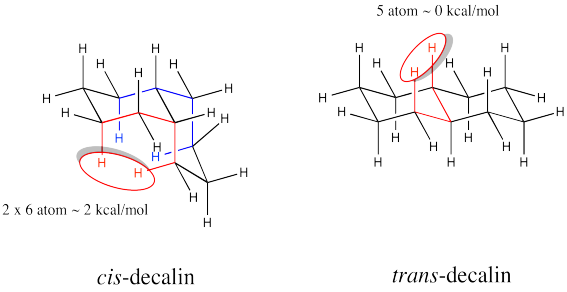
Decalins are examples of fused ring systems. In fused ring systems, two rings share an edge. Decalins contain ten carbons total, but the ten carbons are divided into two rings, each of which is six carbons around.
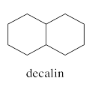
There are two possible isomers of decalin: cis and trans. The easiest way to distinguish the two isomers is to look for the two hydrogens on the shared edge between the two rings. Those two hydrogens are found on the same face of the cis decalin.
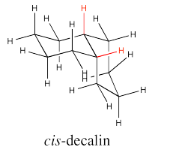
Go to Animation CA11.1. A three-dimensional model of cis-decalin.
The two hydrogens along the junction are seen on the opposite faces in trans decalin.
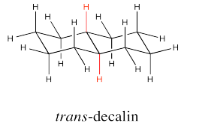
Go to Animation CA11.2. A three-dimensional model of trans-decalin.
Exercise \(\PageIndex{1}\)
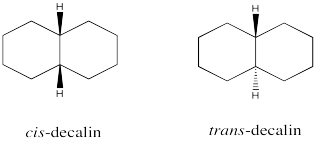
Draw cis-decalin and trans-decalin using a wedge/dash picture.
- Answer
-
Exercise \(\PageIndex{2}\)
Calculate steric strains in cis-decalin and trans-decalin.
- Answer
-
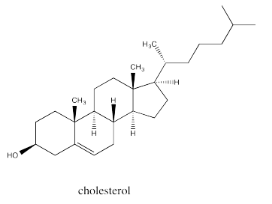
Fused ring systems like decalin are very common. In fact, similar ring systems are found in steroids, which are an important class of lipids. Steroids generally have similar structures that include a series of fused rings as seen in the parent compound, cholesterol.
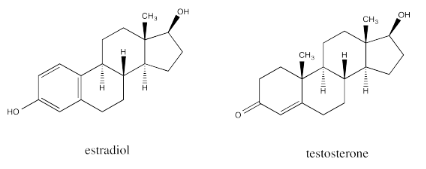
The similarity is striking between estradiol, a female sex hormone, and testosterone, a male sex hormone.
Go to Animation CA11.3. A three-dimensional model of estradiol.
Go to Animation CA11.4. A three-dimensional model of testosterone.
Exercise \(\PageIndex{3}\)
Consider a steroid structure such as cholesterol.
- Based on the relationships along the fused edges between the ring, does the structure more closely resemble that of cis-decalin or that of trans-decalin?
- The overall shape of the structure could probably best be described as (choose one: wide and wavy / compact and curled up / boxy like a cage).
- Answer a:
-
The substituents are always trans along the junctions between each pair of rings. The steroids resemble a series of trans-decalin structures.
- Answer b:
-
The overall structure would be more wide and wavy like a trans-decalin, rather than curled or boxy like a cis-decalin.
Polycyclic systems do not have to be fused along one edge like decalin. They can be connected in a variety of ways. Norbornane is an example of a bicyclic system in which the two rings actually share more than two carbons.
![Skeletal structure of norbornane, systematic name of bicyclo[2,2,1]heptane. Norbornane is composed of two rings fused together, forming a molecule with a total of seven carbons.](https://chem.libretexts.org/@api/deki/files/242984/clipboard_ee6533427871b43bd6eca1695e02e0eb3.png?revision=1)
Norbornane can be used to illustrate the systematic way to describe fused ring systems. The suffix describes the total number of carbons in the ring system. The prefix describes how many rings are fused together. The middle part, in brackets, indicates how many atoms form the "bridges" between the "bridgehead" atoms, where the system splits off into two different rings. One number is given to describe each bridge. In norbornane, there are two 2-carbon bridges and a single 1-carbon bridge.
Exercise \(\PageIndex{4}\)
Write a systematic name for decalin.
- Answer
-
Bicyclo[2.2.0]decane
Exercise \(\PageIndex{5}\)
Provide systematic names for the following structures.
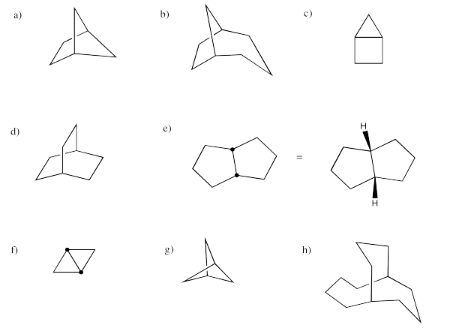
- Answer a:
-
Bicyclo[2.1.1]hexane
- Answer b:
-
Bicyclo[3.2.1]octane
- Answer c:
-
Bicyclo[2.1.0]pentane (more commonly called "housane")
- Answer d:
-
Bicyclo[2.2.2]octane
- Answer e:
-
cis-Bicyclo[3.3.0]octane
- Answer f:
-
cis-Bicyclo[1.1.0]butane
- Answer g:
-
Bicyclo[1.1.1]pentane
- Answer h:
-
Bicyclo[4.3.3]dodecane
Exercise \(\PageIndex{6}\)
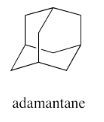
How many different rings can you trace in the structure of adamantane?
- Answer
-
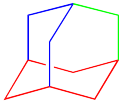
Although we could sketch out many rings using adamantane, just three rings are needed to include all the carbon atoms in the structure. Thus, adamantane is considered a tricyclic system. The systematic nomenclature of tricyclic systems gets a little more complicated, so we won't worry about that.
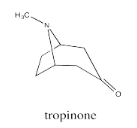
Bicyclic systems are common in nature. For example, the tropane alkaloids all contain a bicyclo[3.2.1] ring system. Alkaloids are a group of natural products that contain basic nitrogen atoms. The tropane alkaloids contain this nitrogen atom in the 1-atom bridge. Tropinone is a simple example.
Common examples of natural products containing the tropinone structure include atropine and cocaine. Atropine, derived from deadly nightshade or belladonna, is a heart medication; it is used to increase heart rate if the rate gets too low. Atropine can also be fatal if used incorrectly, causing a wide range of physiological effects; in some dosages, it can even decrease the heart rate. Cocaine, of course, is a narcotic and a controlled substance.
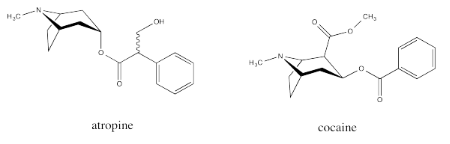
These compounds have a special place in the history of organic and medicinal chemistry. During World War I there was a shortage of atropine, prompting Sir Robert Robinson to develop an efficient synthesis of tropinone as an inexpensive source of synthetic atropine.
Larger Rings
Larger rings of carbon atoms are possible, although they are not as common as five- and six-membered rings. Ring formation is thought to be easiest from a six-carbon chain, because the two ends of the chain can easily wrap around to connect together yet they are not so distant that such an encounter becomes unlikely.
Intermediate-sized rings, such as cyclodecane, become distorted to avoid steric interactions. Internal steric interactions are an added complication in the formation of some rings.
Exercise \(\PageIndex{7}\)
Show the steric interactions within cyclodecane.
- Answer
-
If cyclodecane adopted a regular diamond lattice conformation, there would be a whopping 8 atom interaction in the middle of the ring. That interaction isn't even included in our basis set. It would cost at least 6-7 kcal/mol. As a result, the cyclodecane adopts a twisted structure to avoid this interaction.
At some point, in very large rings, steric problems are not as severe. The hydrogens on the inside of the ring may be far enough away from each other to avoid interactions. Cyclooctadecane is an example of such a ring. Although interior 6-atom interactions are possible, the ring is flexible enough to twist in such a way as to avoid these problems.
Exercise \(\PageIndex{8}\)
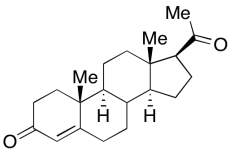
Look at the model for progesterone and make a line drawing of it.
Go to Animation CA11.5. A three-dimensional model of progesterone.
- Answer
-
Exercise \(\PageIndex{9}\)
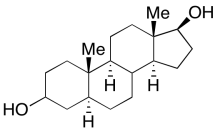
Look at the model for androstanediol and make a line drawing of it.
Go to Animation CA11.6. A three-dimensional model of androstanediol.
- Answer
-
Still pictures of models obtained using Spartan 14 from Wavefunction, Inc., Irvine, California.


