4.12: Organic Functional Groups
- Page ID
- 191167
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Organic compounds are very common in chemistry and biology. Some familiarity with some common types of compounds based on carbon and a few other elements will help you to understand organic chemistry and related fields.
The most common structural piece in organic chemistry is the carbon-carbon bond. Most organic compounds are filled with carbon-carbon bonds. Very often, a network of carbon atoms are single-bonded to each other, and to hydrogen atoms, to build up a complicated structure.
Other pieces, called functional groups, are attached to this framework. Functional groups are specific collections of atoms bonded together in a certain way. These collections of atoms are seen over and over in organic chemistry, and so they are given specific names. This appendix is meant to help you learn to recognize these functional groups. It will also help you learn how to talk about them.
Before looking at functional groups, let's look at simpler compounds that have no functional groups. Alkanes are compounds that contain only carbon and hydrogen, and that contain no double or triple bonds.

Translate the above structures into Lewis - Kekule structures.
Specific alkanes are given names that say something about their structures. Their names end in "ane"; that means they contain only single bonds, not double or triple bonds. A compound that contains no double or triple bonds is sometimes called "saturated". They are sometimes called "paraffinic" hydrocarbons, which means the same thing as saturated hydrocarbons.
The name also contains a part that tells how many carbons are found connected in the chain. These prefixes are used throughout organic chemistry to name other kinds of compounds, so you need to memorize them.
| prefix | # carbons |
|---|---|
| meth | 1 |
| eth | 2 |
| prop | 3 |
| but | 4 |
| pent | 5 |
| hex | 6 |
| hept | 7 |
| oct | 8 |
| non | 9 |
| dec | 10 |
Provide names for the following alkanes.

- Answer a
- Answer b
- Answer c
Answer apropaneAnswer bpentaneAnswer chexane
The chain of carbons in an alkane is called an alkyl chain. Sometimes there are smaller alkyl chains attached to the main chain of an alkane. The same prefixes can be used to tell how many carbons are in these smaller branches.
Numbers are used to count how far along the chain these branches occur. You need to start on the end of the chain and count the carbons until you get to the place where the branch occurs. If there are two different possible chains, choose the longest chain as the base name. If there are two different directions possible, start at the one that gives the lowest numbers for the branches.
If there are two different branches of the same size on a chain, you need to say so. A different prefix is used to say how many of the same piece are present.
| prefix | # of groups |
|---|---|
| di | 2 |
| tri | 3 |
| tetra | 4 |
| penta | 5 |
Provide names for the following branched alkanes.

- Answer a
- Answer b
- Answer c
- Answer d
- Answer e
- Answer f
Answer a3-methylhexaneAnswer b2,2-dimethylpentaneAnswer c2,3-dimethylbutaneAnswer d2,2,3,3-tetramethylpentaneAnswer e3,5-dimethylheptaneAnswer f4-ethyl-3,6-dimethyloctane
"Aliphatic hydrocarbons" specifically refers to branched chains of saturated hydrocarbons. "Alicyclic hydrocarbons" are very similar but they contain rings of carbons. If a chain of carbons wraps around to form a ring, the prefix "cyclo" is used.
All these elements will be used in naming other compounds as well.
Provide names for the following cyclic alkanes.
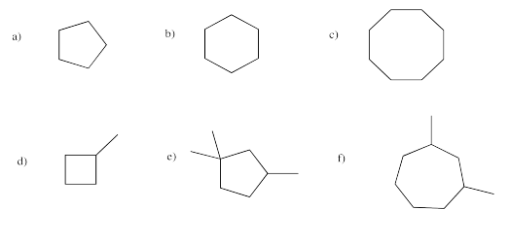
- Answer a
- Answer b
- Answer c
- Answer d
- Answer e
- Answer f
Answer acyclopentaneAnswer bcyclohexaneAnswer ccyclooctaneAnswer dmethylcyclobutaneAnswer e1,1,3-trimethylcyclopentaneAnswer f1,3-dimethylcycloheptane
Homoatomic functional groups
Any feature other than an alkyl chain is called a "functional group". A functional group is also just a place where reactions take place.
Homoatomic functional groups contain only carbon and hydrogen. They differ from alkyl groups only in having multiple C-C bonding.
The C=C functional group is called an alkene. Simple compounds that contain C=C double bonds are also called alkenes. The term "olefin" also means alkene, and "unsaturated" or "olefinic" hydrocarbons contain double bonds.

In naming alkenes, the suffix "ene" is used instead of "ane". The base name of the alkene describes the length of the chain containing the double bond. A number says how far the double bond is from the nearest end of the chain.
Provide names for the following alkenes.

- Answer a
- Answer b
- Answer c
- Answer d
Answer a1-hexeneAnswer b2-methyl-2-penteneAnswer c1-methylcyclohexeneAnswer d2,4,6-trimethyl-2-heptene
The C-C triple bond is called an alkyne. "Acetylenic" hydrocarbons or alkynes are compounds that contain C-C triple bonds. These compounds are also examples of "unsaturated hydrocarbons".

Provide names for the following hydrocarbons.

- Answer a
- Answer b
- Answer c
- Answer d
- Answer e
Answer acyclopenteneAnswer b1,1-dimethylcyclohexaneAnswer c3-hexyneAnswer d4-methylcyclohexeneAnswer e1-hexyne
Describe the geometries of the following carbons:
a) any carbon in pentane b) the second carbon in 2-butene c) the first carbon in 1-hexyne
- Answer a
-
tetrahedral
- Answer b
-
trigonal planar
- Answer c
-
linear
Aromatic compounds appear to contain C-C double bonds but they are very different from alkenes. The most common aromatic is benzene. Because benzene is a very common structural piece, you should be familiar with some of the language associated with it.

Benzene rings with two groups attached are common enough that different terms are used to describe their isomers. The terms ortho-, meta- and para- are used only to describe the relationship between two groups around benzene, and not any other compounds. These terms are sometimes abbreviated to o-, m- or p-. Alternatively, two groups attached to a benzene can simply be numbered in order to make it clear where they are. If more than two groups are attached, numbering is used; terms such as "ortho" no longer apply.
Note that a benzene group is sometimes called a phenyl group. It is not called a benzyl group, nor a benzoyl group. Those two groups both contain a phenyl ring but they are not exactly the same.
Provide names for the following aromatics.

- Answer a
- Answer b
- Answer c
- Answer d
- Answer e
- Answer f
Answer amethylbenzeneAnswer bpropylbenzeneAnswer c1,2-dimethylbenzene or o-dimethylbenzene (also o-xylene)Answer d1,3-dimethylbenzene or m-dimethylbenzene (also m-xylene)Answer e1,4-diethylbenzene or p-diethylbenzeneAnswer f2-ethyl-1,4-dimethylbenzene
Heteroatomic Functional Groups: Carbonyls
Heteroatomic functional groups contain atoms other than carbon and hydrogen.
Probably the most important set of heteroatomic functional groups is the set that contains carbon-oxygen double bonds. The C=O group is called a carbonyl (carbon-EEL). Carbonyl compounds all contain the carbonyl group.
There are two subsets of carbonyl compounds: regular carbonyls and heteroatom-substituted carbonyls. In regular carbonyls, the carbon in the C=O group is attached only to carbon or hydrogen. It is not attached to additional heteroatoms such as nitrogen or oxygens.
There are two kinds of regular carbonyls. In ketones, the carbonyl carbon is attached only to other carbons. In aldehydes, the carbonyl carbon is attached to a hydrogen atom as well as a carbon.

Aldehydes and ketones have specific naming conventions. The suffix for the name of an aldehyde is "-al"; whereas the suffix for the name of a ketone is "-one". In the case of a ketone, the location of the carbonyl along the chain can be described with a number. Otherwise, the names of aldehydes and ketones follow the rules we have seen for other organic compounds.
Provide names for the following compounds.

- Answer a
- Answer b
- Answer c
- Answer d
Answer a2,2-dimethylhexanalAnswer b2-methylcyclopentanoneAnswer c3-nonanoneAnswer d2,4-dimethyl-2-hexenal
Heteroatom-substituted carbonyls all have a heteroatom attached to the carbonyl carbon. A heteroatom is an atom other than hydrogen or carbon, such as nitrogen, oxygen or chlorine. Heteroatom-substituted carbonyls are often called "carboxylic acid derivatives" or sometimes "carboxyloids".

In carboxylic acids, the carbonyl carbon is attached to an OH group. The OH group is often called a hydroxyl group. Fatty acids and amino acids are examples of biological compounds that contain carboxylic acids.
Two biologically important carboxyloids are amides and esters
In esters, the carbonyl carbon is attached to an oxygen, like in a carboxylic acid. However, the oxygen is not attached to a hydrogen. The oxygen is attached to a carbon. The properties of esters differ enough from carboxylic acids that they are given a different name. Glycerides are biological compounds that contain ester groups. Glycerides are found in fats.
In amides, the carbonyl carbon is attached to a nitrogen. Unlike esters and carboxylic acids, it does not matter whether the nitrogen is attached to a hydrogen or to another carbon. The presence of a hydrogen on the nitrogen changes the properties of the amide less dramatically than in esters and carboxylic acids.
Because the amide does change subtly if there is a hydrogen attached to the nitrogen, there is a way to describe the presence ar absence of hydrogens. An amide with two hydrogens on the nitrogen is called a primary amide. The nitrogen is connected to only one (Latin for first: primus) carbon: the carbonyl carbon.
An amide with two carbons connected to the nitrogen is called a secondary amide (Latin for second: secondus). An amide with three carbons connected to the nitrogen is called a tertiary amide (Latin for third: tertius).
Naming simple examples of these compounds, as before, involves a change in the suffix of the name. The suffix for a carboxylic acid is "-oic acid". Esters really have a two-part name. The first part describes the group attached to the enchained oxygen atom of the ester, with the suffix "-yl". The second part describes the portion that contains the carbonyl, with the suffix "-oate". For an amide, the suffix is "amide". However, other groups attached to the nitrogen are usually prefixed with "N-"; this is a little like numbering the position of a group in previous examples, but this time it underscores that the group is attached to a nitrogen.
Provide names for the following carboxyloids.

- Answer a
- Answer b
- Answer c
- Answer d
Answer abutyl propanoateAnswer bN,N-diethylbutanamideAnswer c6-methylheptanoic acidAnswer d4-pentenoic acid
A third biologically important carboxylic acid derivative contains a sulfur attached to the carbonyl carbon. Usually the sulfur is attached to another carbon as well. Because sulfur is in the same group in the periodic table as oxygen, this functional group is similar to an ester. It is called a thioester. It is much less common than amides and esters. A biological example of a thioester is acetyl conezyme A, which plays an important role in many biological reactions. In fact, the thioester plays the key role in the reactions of acetyl coenzyme A.
Naming thioesters is just like naming other esters, except that the prefix "thio-" is inserted into the second part of the name.

Two other important carboxylic acid derivatives are not normally seen in biology. They are important because they are used synthetically or industrially to make the other carboxylic acid derivatives.
Acid anhydrides are prepared by heating carboxylic acids at high temperatures until water is lost. Acid anhydrides contain two carbonyl units, connected by an oxygen. Usually those two units are the same as each other. The name for an anhydride indicates the number of carbons in just one of those pieces (since the other one is the same) with the suffix "-oic (space) anhydride".
Acid chlorides have a chlorine atom attached to the carbonyl carbon. The name for an acid chloride contains the suffix "-oyl (space) chloride". Other acid halides (such as acid fluorides) are known, but they are less common than acid chlorides.
Simple Heteroatomic Functional Groups (No Carbonyls)
There are several heteroatomic functional groups that do not contain carbonyls.
Alcohols contain an OH or hydroxyl group, but the hydroxyl is not attached to a carbonyl.

Sometimes alcohols are classed into sub-groups, as "primary", "secondary" or "tertiary". Those words just describe the carbon attached to the OH group. How many carbons are attached, in turn, to that carbon? Are there two, three, or just one? Those classifications have some influence on how reactive the alcohol will be under different conditions.
The suffix in the name of an alcohol is "ol". It does not matter whether the alcohol is described as a primary, secondary or tertiary one They are still named according to the same rule.
Phenols are alcohols in which the hydroxyl group is directly attached to a phenyl group, or benzene ring. Their behavior is different enough from other alcohols that they are sometimes thought of as a separate group. For one thing, they are somewhat acidic. Coming into contact with phenols, whether they are found in pine resin or, in a much more extreme case, poison ivy, can cause severe itching in the skin. Regular alcohols are much less likely to cause that reaction.
Ethers are kind of like alcohols in that they contain oxygen atoms. Ethers contain a C-O-C group, but neither carbon is part of a carbonyl. Generally, to name an ether, we list the two parts attached to the oxygen atom, and follow those two names with "ether".

Amines contain nitrogen atoms connected to three different atoms, but not connected to a carbonyl. If the nitrogen is attached only to one carbon, the functional group is a primary amine. If it is attached to two carbons, it is a secondary amine. If it is attached to three carbons, it is a tertiary amine. This classification system is a little bit different from the one used with alcohols, in that it refers to the number of things attached to the nitrogen itself. In oxygen compounds, the number of things attached to the oxygen determined whether we called it an alcohol or an ether.
Naming amines is a little like naming ethers. We list the groups attached to the nitrogen, followed by "amine". Usually these groups are listed in alphabetical order. Sometimes, as with amides, the idea that a group is attached to the nitrogen is usually reinforced with the prefix "N-".

Thiols and thioethers are the sulfur analogues of alcohols and ethers. Phosphines are the phosphorus analogues of amines. None of these groups are as common as alcohols, ethers and amines. The sulfur-containing compounds are named in a similar way to their oxygen analogues, but with the suffix "-thioether" or "-thiol" used instead of "-ether" or "-ol". Similarly, phosphines have the suffix "-phosphine" instead of "-amine".

Alkyl halides, or haloalkanes, are compounds that contain a halogen that is not attached to a carbonyl. These compounds are not common biologically, but they are important industrially. Usually halogens are treated like alkyl groups in terms of naming compounds that contain them. A halogen is described as a prefix ending in "-o", rather than the prefix ending in "-yl" that is used with alkyl groups. For example, an alkyl bromide is named as a bromoalkane, such as bromoethane or bromohexane.

There are a few other functional groups that contain multiple bonds to nitrogen. An imine is the nitrogen analogue of an aldehyde. An imine contains a carbon-nitrogen double bond. The carbon almost always has a hydrogen attached as well. The word "imine" is just appended to the name of the related aldehyde to describe a specific imine.
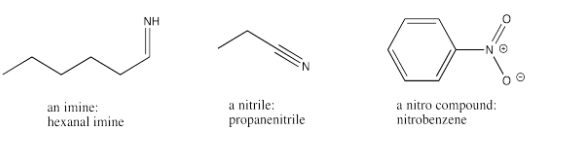
A nitrile contains a carbon-nitrogen triple bond. The suffix for a nitrile is just "-nitrile".
A nitro group contains the NO2 unit. "Nitro-" is usually added as a prefix to signal the presence of this group in a compound.
| fun group | suffix |
|---|---|
| none | ane |
| alkene | ene |
| alkyne | yne |
| ketone | one |
| aldehyde | al |
| carboxylic acid | oic acid |
| ester | -yl ...-oate |
| amide | amide |
| thioester |
Provide names for each of the following compounds.

- Answer a
- Answer b
- Answer c
- Answer d
- Answer e
- Answer f
- Answer g
- Answer i
- Answer j
- Answer k
- Answer l
- Answer m
- Answer n
Answer a1-chloro-2-methylcyclohexaneAnswer bcyclooctanolAnswer cethyl cyclopentyl etherAnswer dN-propylcyclohexylamineAnswer e5,5-dimethylhelpan-2-olAnswer f3-bromo-4,4-dimethyloctaneAnswer gdibutylamineAnswer imethyl phenyl ether (or anisole)Answer jethane thiolAnswer kdiethyl thioetherAnswer ltriethylphosphineAnswer mbutanenitrileAnswer nnitromethaneNote that sometimes a number is located directly in front of the suffix for the group to which it refers.
Each of the following compounds contains three functional groups. Identify them.

- Answer a
- Answer b
- Answer c
- Answer d
- Answer e
Answer abenzene (or aromatic), ketone, and etherAnswer bbromide, amine, and aldehydeAnswer calcohol, thiol, and esterAnswer dthioester, amide, and alkeneAnswer ealkyne, alcohol, and carboxylic acid
Identify the circled functional groups.

- Answer

Identify the circled functional groups (more advanced level).

- Answer



