4.11: Controversial Lewis Structures
- Page ID
- 191166
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There is sometimes controversy in science. Argument can be an important part of how we arrive at a better understanding of things. In the context of molecules and structures, there have been long-running controversies about how to draw certain phosphorus and sulfur compounds. A look at that controversy can tell us some things about how different chemists think about bonding.
The controversy concerns how to draw, and think about, the bonding in a number of "high-valent" sulfur and phosphorus compounds, in which the sulfur or phosphorus atom is bonded to more than the usual number of neighbors.
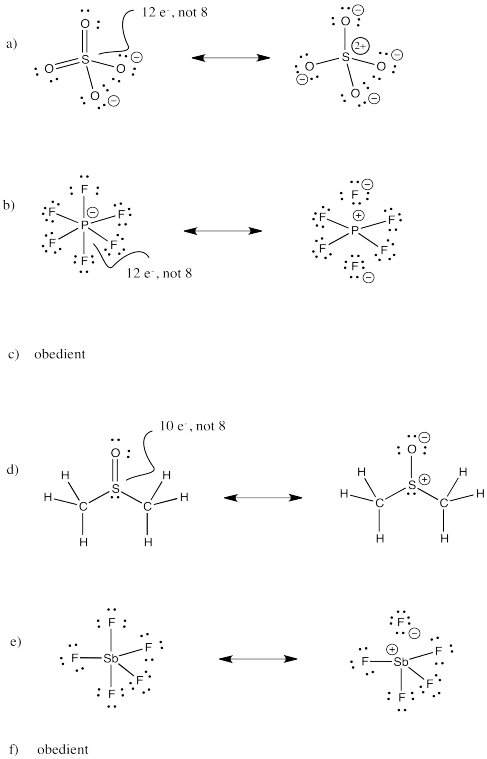
Sulfate ion, SO42-, is an example of a high-valent sulfur compound. Sulfur, like oxygen, frequently forms two bonds. In sulfate, the sulfur is attached to four different atoms.
We could draw that structure in two ways. A structure that obeys the octet rule would have a single bond to each oxygen. That would satisfy the octet of sulfur. In order to fill in the right number of electrons for this group of atoms, each oxygen will have a negative formal charge. Sulfur will have a charge of plus two.
A different way to draw the structure would include double bonds between sulfur and two of the oxygen atoms. That structure would have less charge separation than the other structure. However, the sulfur would have twelve electrons in its valence shell, not eight. It would exceed its octet.
Can an atom have more than eight electrons in its valence shell? Lots of them can, if they are further down the periodic table. Whether sulfur and phosphorus can is much less clear. Frequently, the fact that these are 3rd row atoms is considered significant. After all, the d electrons begin in the third row. Perhaps sulfur and phosphorus can accommodate extra electrons because they have d orbitals. The d orbitals don't have any electrons in them, yet, so maybe they can accept some from other aroms via bonds.
Many theoretical chemists (including experts in sulfur and phosphorus chemistry) are convinced that this scenario just isn't possible. The d level in these atoms is just too high in energy. Other atoms can't possibly donate electrons to sulfur and phosphorus in this way.
And yet, many chemists (including experts in sulfur and phosphorus chemistry) still draw sulfate with double bonds. Why would they make such a mistake in the face of reason?
Partly, it has to do with experimental evidence. Remember, "experimental" sounds wishy-washy and tentative to non-scientists, but to a chemist the term really means "reality-based". One of the reasons people draw double bonds in many sulfur and phosphorus compounds is that the bonds simply behave like double bonds. That is, they are stronger and shorter than single bonds. We might not think about those bonds in exactly the way we think about double bonds in other situations, but the experimental evidence says that there is some additional attraction between the sulfur and oxygen atoms in these cases.
There is additional evidence that sulfur and phosphorus can "exceed the octet" or form "Lewis-disobedient" structures. Compounds like phosphorus pentafluoride (PF5) clearly require more than four bonds to sulfur. Without five bonds, we would need to draw one of the fluorides as an anion. It's certainly possible that this could be the true structure, but there is evidence from X-ray crystallography that all of the fluorines are attached to phosphorus in PF5, as well as in the related PF6- anion (hexafluorophosphate, a very common molecular ion in X-ray crystallographic studies).
So, sulfur and phosphorus really can form additional bonds. The only true controversy about the octet rule in sulfur and phosphorus chemistry is a little too subtle to explore very deeply at this point: it's whether sulfur can form a double bond in these cases. The issue has to do with ideas from molecular orbital theory, which is a later chapter, and how well d orbitals can form "pi bonds" with the p orbital on oxygen.
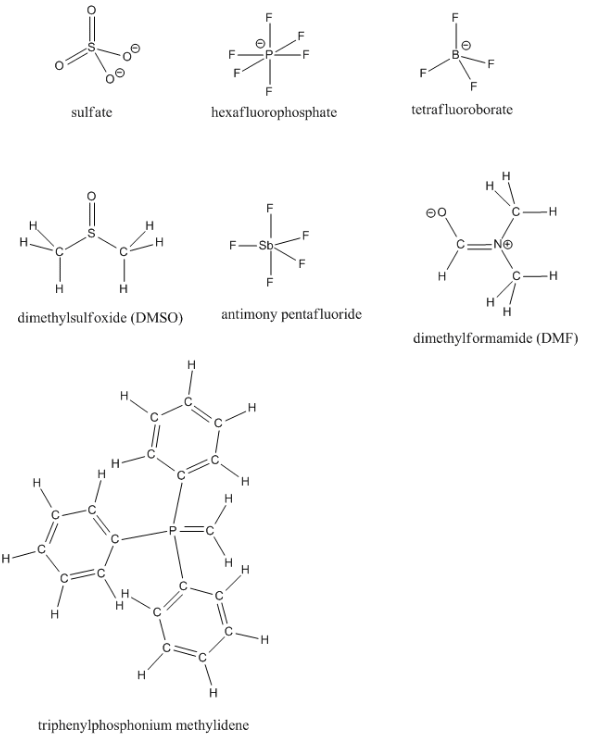
Fill in any missing lone pairs on the following structures.
- Answer
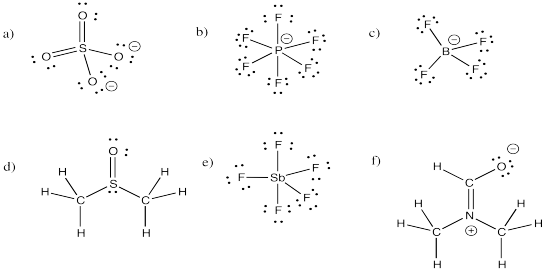
Indicate whether each of the structures in Exercise \(\PageIndex{1}\) (IM11.1) obeys the octet rule. If not, provide a resonance structure that obeys the octet rule.
- Answer
-