1.7: Atoms- Solutions to Selected Problems
- Page ID
- 190583
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exercise 1.1.3
- one atom of mercury with one atom of oxygen.
- two atoms of mercury with one atom of oxygen.
- 200 amu (Hg) + 16 amu (O) = 216 amu (HgO)
- 2 x 200 amu (Hg) + 16 amu (O) = 416 amu (HgO)
Exercise 1.1.4
- 200 g (Hg) + 16 g (O) = 216 g (HgO)
- 2 x 200 g (Hg) + 16 g (O) = 416 g (Hg2O)
- If one mole is 216 g of HgO, then 0.25 mole must be a quarter of that amount, or 54 g.
\[ 0.25 mol \times 216 \frac{g}{mol} = 54 g \nonumber\]
Alternatively written as
\[ 0.25 mol \times 216 g \: mol^{-1} = 54 g \nonumber\]
The amount of mercury is just a fraction of that: 200/216. So the answer is \(\frac{200}{216} \times 54 g = 50 g\).
d) What fraction of a mole is 2.08 g of Hg2O, if one mole is 416 g?
\[ \frac{2.08g}{416 g \: mol^{-1}} = 0.005 mol \nonumber\]
Notice that when we divide g by g mol-1, the grams cancel and the mol-1 becomes mol.
There is 1 mol of O in 1 mol of HgO, so 0.005 mol of O are needed for 0.005 mol HgO.
\(0.005 mol \times 16 g \: mol^{-1} = 0.08 g\) O needed
Exercise 1.1.6
a) sulfur b) silicon c) calcium d) potassium and lithium
e) phosphorus f) iodine g) silver and copper h) ruthenium
Exercise 1.1.7
a) 20 b) Na c) 16.00 d) phosphorus
e) carbon f) Sn g) 32.07 h) potassium
Exercise 1.1.8
a) Ne: 20 g/mol b) Fe: 56 g/mol c) Cu: 64 g/mol d) Au: 197 g/mol e) Si: 28 g/mol
Exercise 1.1.9
- H2O: \(18 \frac{g}{mol} (2 \times H + 1 \times O = 2 \times 1 + 1 \times 16 \frac{g}{mol})\)
- NaHCO3 : \(84 \frac{g}{mol} (3 \times O + 1 \times Na + 1 \times H + 1 \times C = 3 \times 16 + 1 \times 23 + 1 \times 1 + 1 \times 12 \frac{g}{mol})\)
- SiO2 : \(60 \frac{g}{mol} (1 \times Si + 2 \times O = 1 \times 28 + 2 \times 16 \frac{g}{mol})\)
- NaCl : \(58 \frac{g}{mol} (1 \times Na + 1 \times Cl = 1 \times 23 + 1 \times 35 \frac{g}{mol})\)
- TaN : \(195 \frac{g}{mol} (1 \times Ta + 1 \times N = 1 \times 181 + 1 \times 14 \frac{g}{mol})\)
- NH3 : \(17 \frac{g}{mol} (1 \times N + 3 \times H = 1 \times 14 + 3 \times 1 \frac{g}{mol})\)
- NaH2PO4 : \(120 \frac{g}{mol} (1 \times Na + 2 \times H + 1 \times P = 4 \times O = 1 \times 23 + 2 \times 1 + 1 \times 31 + 4 \times 16 \frac{g}{mol})\)
- CH3CO2H : \(60 \frac{g}{mol} (2 \times C + 4 \times H + 2 \times O = 2 \times 12 + 4 \times 1 + 2 \times 16 \frac{g}{mol})\)
Tthe molar weight of the atom was rounded to the nearest gram. In order to be very careful and avoid "error propagation", the more exact molar weight (to several decimal places) could be used and rounding could be performed after the calculation. In these cases, the result would be the same. The result could be different if very large numbers of atoms were found in the compound.
Exercise 1.2.1
a) O: 8 protons, 8 electrons, 8 neutrons
The atom is neutral overall, so the number of positively charged protons is equal to the number of negatively charged electrons. The atomic weight is provided almost entirely by the protons and neutrons, so the number of protons plus number of neutrons equals the atomic weight.
b) P: 15 protons, 15 electrons, 16 neutrons
c) Zn: 30 protons, 30 electrons, 35 neutrons
d) Au: 79 protons, 79 electrons, 118 neutrons
Exercise 1.2.3
Carbon: \(\frac{(99 \times 12amu) + (1 \times 13 amu)}{100} = \frac{1188 + 13amu}{100} = \frac{1201amu}{100} = 12.01 amu\)
Exercise 1.2.4
The negatively charged electron's departure from the nucleus leaves behind a positive charge. A neutron is converted into a proton. The overall atomic weight remains the same, but the atom ends up with one more proton and one more electron. A 14C is converted into a 14N.
Exercise 1.2.6
Suppose y is the decimal fraction of 35Cl and z is the decimal fraction of 37Cl.
\(35.5 amu = \frac{(y \times 35 amu + z \times 37 amu)}{100}\) but y + z = 1
\[35.5 amu = (y \times 35 amu + (1-y) \times 37 amu) \nonumber\]
\[35.5 amu = y \times 35 amu - y \times 37 amu + 37 amu \nonumber\]
\[(37-35) \times y amu = 37 amu - 35.5 amu \nonumber\]
\[2 \times y amu = 1.5 amu \nonumber\]
\[y = 0.75 (75 \% ^{35}Cl) \nonumber\]
\[z = 0.25 (25 \% ^{37}Cl \nonumber\]
Exercise 1.2.7
- 1, 2, 3, 4, 5... a series of whole numbers, or n.
- 2, 4, 6, 8, 10... a series of even numbers, or 2n (because every even number is two times another number).
- 3, 5, 7, 9, 11... a series of odd numbers, or 2n + 1 (because every odd number is one more than some even number).
- 1, 4, 9, 16, 25... a series of squares, or n2.
- 2, 4, 8, 16, 32... a series in which each numer is double the last number, or 2n.
- 1, 1/2, 1/4, 1/9, 1/16... a series of reciprocals of squares, or 1/n2.
Exercise 1.2.8
- When q increases, F increases. The increase is linear: if q1 doubles, F doubles. As the charge in the nucleus gets larger, the force of attraction gets larger.
- When r increases, F decreases. The decrease is nonlinear: if r doubles, F drops by a factor of four, rather than a factor of two. As the distance from the nucleus gets longer, the attraction to the nucleus drops sharply.
- Hydrogen and helium are both in the first row of the periodic table. To a rough approximation, the distance between nucleus and electron is similar in these two atoms. However, helium has a charge of 2+ in its nucleas, compared to the 1+ charge in the nucleus of a hydrogen atom. As a result, the attraction of an electron on helium to the nucleus would be about twice as great as the attraction of an electron on hydrogen to its nucleus.
Helium's electrons are much more tightly held than hydrogen's.
The situation is really much more complicated than that. For example, if helium's electrons are more tightly attracted to the nucleus than hydrogen's, then helium's electrons ought to be pulled closer to the nucleus than hydrogen's. That means helium's electrons are held even more tightly than we at first thought.
Another complicating factor is that helium has two electrons, whereas hydrogen has only one. An electron may be attracted to the nucleus, but electrons repel each other. That second electron in helium should offset the extra attraction to helium's more positive nucleus. That means helium's electrons may be less tightly attracted than we originally thought.
However, the effect of the second electron is much smaller than it first appears. That's because the second electron could be anywhere around the helium atom. It has a 50% chance of being further away from the first electron than that positively charged nucleus. The farther it is away, the smaller its influence. Helium's electrons are definitely more strongly attracted to the nucleus than are hydrogen's, but it is difficult to say exactly how much more without the help of some more sophisticated tools.
Exercise 1.2.9
- As wavelength gets longer (value of λ increases), energy decreases.
- As frequency gets higher (value of ν increases), energy increases.
Exercise 1.2.10
| Element Symbol | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons | Charge |
|---|---|---|---|---|---|---|
| H | 1 | 1 | 1 | 0 | 1 | 0 |
| H | 1 | 1 | 1 | 0 | 0 | +1 |
| H | 1 | 1 | 1 | 0 | 2 | -1 |
| H | 1 | 2 | 1 | 1 | 1 | 0 |
| H | 1 | 3 | 1 | 2 | 1 | 0 |
| Be | 4 | 9 | 4 | 5 | 2 | +2 |
| C | 6 | 12 | 6 | 6 | 6 | 0 |
| Mg | 12 | 25 | 12 | 13 | 12 | 0 |
| Tc | 43 | 98 | 43 | 55 | 43 | 0 |
| Ca | 20 | 40 | 20 | 20 | 18 | +2 |
| Si | 14 | 28 | 14 | 14 | 14 | 0 |
| K | 19 | 47 | 19 | 28 | 15 | +4 |
| Fe | 26 | 56 | 26 | 30 | 23 | +3 |
| Br | 35 | 79 | 35 | 44 | 36 | -1 |
| Ti | 22 | 39 | 22 | 17 | 21 | +1 |
| P | 15 | 30 | 15 | 15 | 15 | 0 |
| Al | 13 | 27 | 13 | 14 | 10 | +3 |
| S | 16 | 32 | 16 | 16 | 16 | 0 |
| Pd | 46 | 106 | 46 | 60 | 45 | +1 |
| Cr | 24 | 52 | 24 | 28 | 21 | +3 |
| Sn | 50 | 118 | 50 | 68 | 50 | 0 |
| Hg | 80 | 200 | 80 | 120 | 79 | +1 |
| Au | 79 | 197 | 79 | 118 | 78 | +1 |
Exercise 1.3.1
- The energy of the electron gets higher.
- The energy of the electron gets lower.
- The wavelength gets shorter.
- The wavelength gets longer.
Exercise 1.3.2
The probability of finding an electron at a node is zero.
Exercise 1.3.3
Your drawing is beautiful. You should put it on your fridge, or else send it to your mother.
Exercise 1.4.1
a) 2s b) 3p c) 3d e) 5f e) 4s
Exercise 1.4.2
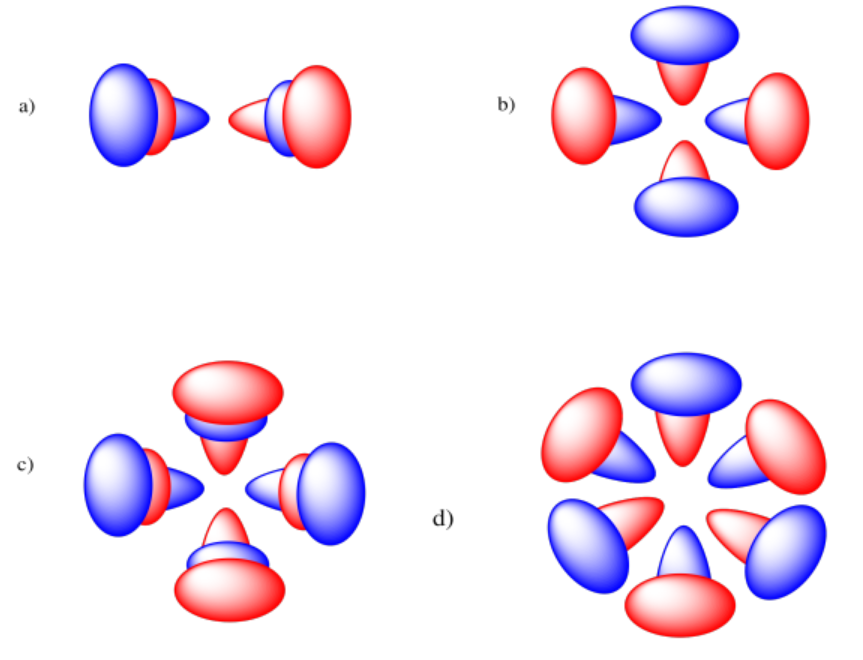
Exercise 1.4.3
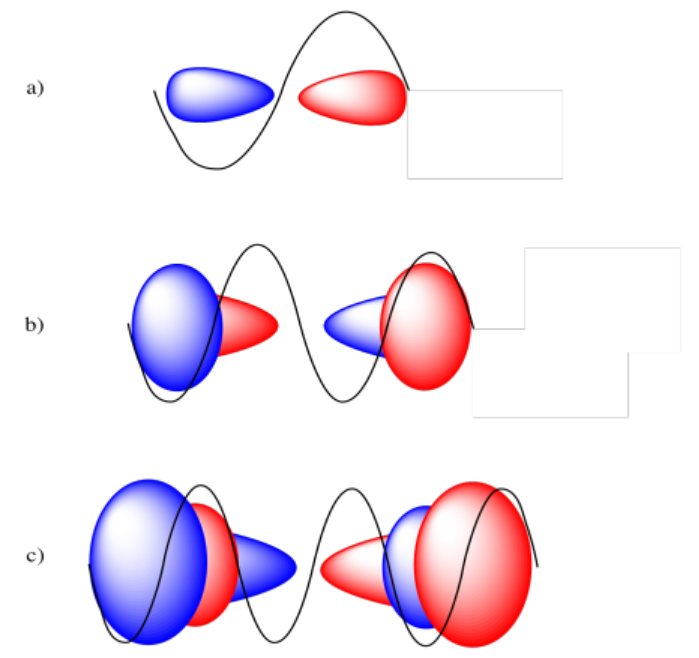
From one end of the orbital to another, the 2p orbital covers a full sine wave, a 3p orbital covers two full sine waves, and a 4p orbital covers three full sine waves.
Exercise 1.5.1
- O: 1s22s22px22py12pz1
- S: 1s22s22p63s23px23px23py13pz1
- Si: 1s22s22p63s23px23px13py1
- N: 1s22s22px12py12pz1
- Ar: 1s22s22p63s23p6
- Ne: 1s22s22p6
Exercise 1.5.2
- Cl: [Ne]3s23px23py23pz1
- Ca: [Ar]4s2
- Al: [Ne]3s23px1
- P: [Ne]3s23px13py13pz1
Exercise 1.5.3
- iron, Fe: [Ar]4s23d6
- nickel, Ni: [Ar]4s23d8
- mercury, Hg: [Xe]6s24f145d10
- lead, Pb: [Xe]6s24f145d106p2
- arsenic, As: [Ar]4s23d104p3
- titanium, Ti: [Ar]4s23d2
Exercise 1.5.4
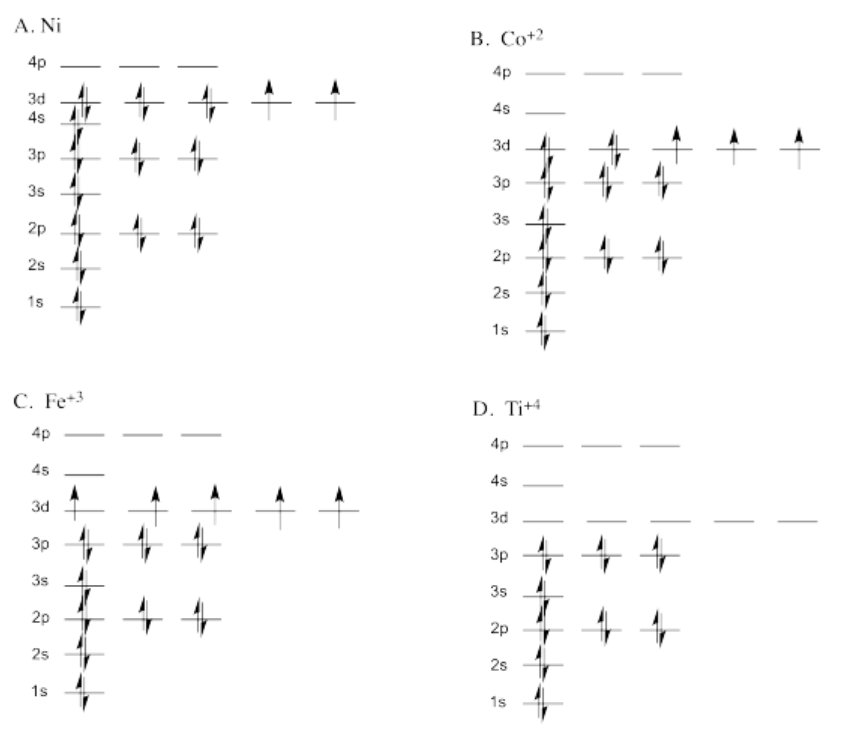
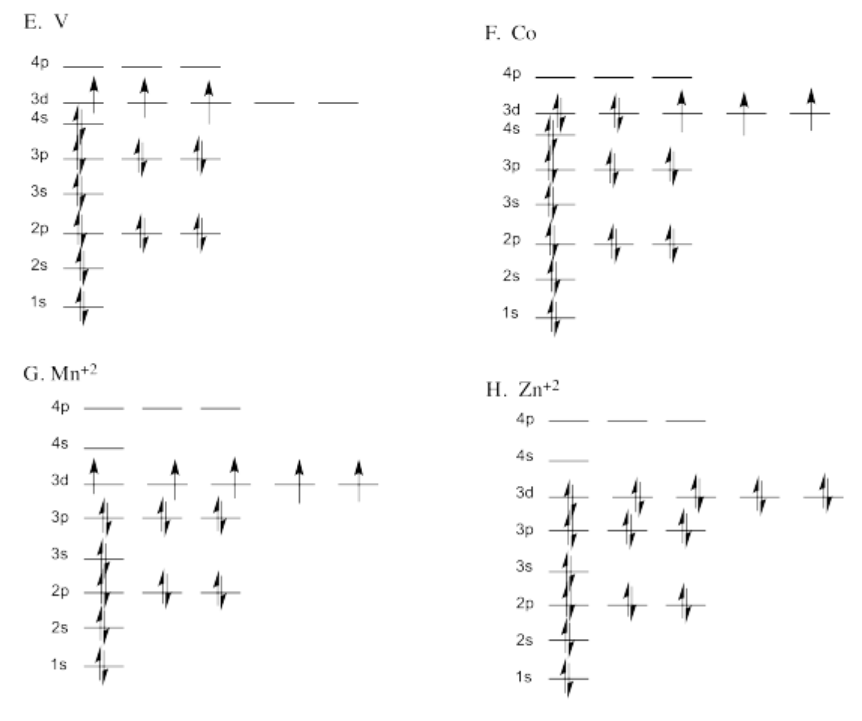
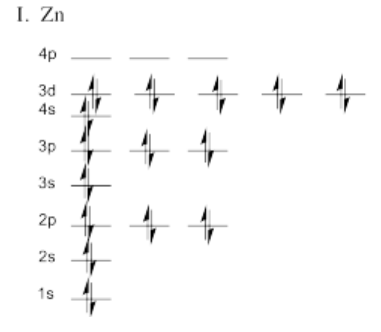
Exercise 1.6.1
- Zn: Cd, Hg
- Ca: Mg, Ba
- O: S, Se
- Cl: F, Br
- Cr: Mo, W
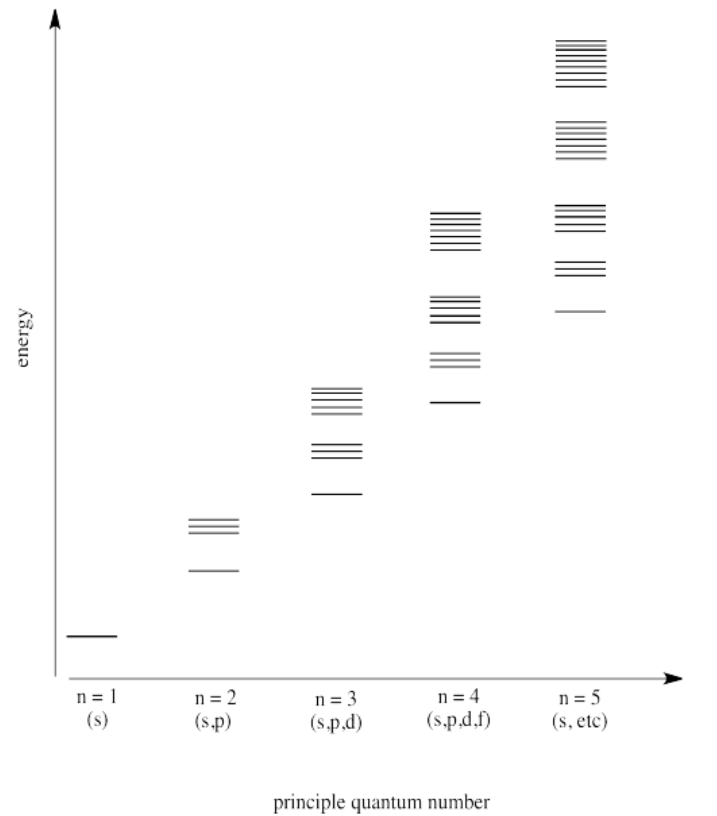
Exercise 1.6.2
Exercise 1.6.3
The atomic number is the number of protons in the nucleus. For two atoms in the same row of the periodic table, the outermost electrons are roughly the same distance away from the nucelus. The more positive protons there are in the nucleus, the more tighly held are the electrons.
Exercise 1.6.4
Take it from the boron. The oxygen atom is holding its electrons much more tightly.
Exercise 1.6.5
According to the drawing, the neon would take the electron, because of all the atoms depicted in the graph, neon attracts electrons most strongly. However, there is a complication. Although neon strongly attracts its own electrons, it can't accommodate an extra electron as easily as could fluorine, the next-best candidate. In "Lewis" terms, neon has a "full octet". In quantum terms, an additional electron would have a higher principle quantum number and be placed in the next "shell", farther from the nucleus. With spin-pairing, fluorine can accept another electron into its valence shell.
Exercise 1.6.6
The oxygen would pull the electrons in the bond more tightly to itself.
Exercise 1.6.7
Moving from one row to the next in the periodic table signifies that the outermost electron is in a shell farther from the nucleus. Those outermost electrons are less tightly held if they are farther from the nucleus.
Exercise 1.6.8
- Cs+ F-
- Na+ O-
- K+ H-
Exercise 1.6.9
The attraction for an electron falls off with 1/r2. As the value of r gets larger and larger, the quantity 1/r2 will begin to approach a limit (of zero). As a result, the difference between two successive values of 1/r2 in a series gets smaller and smaller.
Exercise 1.6.10
- Mg > Ca
- Sn > Pb
- Sb > Ag
- As > Ga
- Cu > W
- S > Tl
Exercise 1.6.11
- The electron is held by its attraction to the nucleus.
- As the number of protons in the nucleus increases, the electron becomes more tightly held, and harder to remove.
The relationship is similar to the one seen for electronegativity, and not coincidentally. Electronegativity can be calculated in a number of ways, but one of those ways uses the ionization energy as a factor.
Electronegativity is a calculated value, whereas ionization energy is an experimentally determined one. This difference brings up an important philosophical distinction. To a beginning student, an experimental value seems faulty, whereas a calculated value sounds good. To an experienced chemist, an experimental value is verifiable; it is real. In contrast, a calculated value is enhanced in prestige only if it can be shown to agree with experiment.
Exercise 1.6.12
In order to escape the atom, an electron must gain energy. As shown in problem AT5.2., the 2p energy level is higher than the 2s energy level. That means a 2p electron already has more energy than a 2s electron. The 2p electron will not need as much additional energy in order to escape from the atom. As a result, boron's ionization energy is a little lower than beryllium's.
The additional protons in the nucleus of carbon and nitrogen more than make up for that effect.
In the case of oxygen, the next electron is just added to a 2p level, but in this case it must be paired with another electron in the same region of space (in the same "orbital"). The repulsion between these electrons, or "pairing energy", slightly destabilizes the oxygen, so less energy will be needed to remove an electron.
Once again, the continued addition of extra protons eventually compensates for this pairing effect.
Exercise 1.6.13
As with electronegativity, ionization energy decreases as the distance to the nucleus increases because of the 1/r2 relationship in Coulomb's Law.
Exercise 1.6.14
- Energy is released because of the attraction of the free electron to a nucleus. The electron moves to lower energy as it becomes stabilized by its interaction with the nucleus.
- The electron can get much closer to the hydrogen nucleus thanto the lithium nucleus, and so on. More energy will be released owing to the strong interaction between the electron and the hydrogen nucleus compared to the interaction between the electron and the lithium (or sodium or potassium...) nucleus.
Exercise 1.6.15
- The data zig-zags, but broadly speaking there is an increase in electron affinity as the number of protons in the nucleus increases.
- Beryllium and neon have zero electron affinity because the next electron in each case would be added to a higher energy level. In the case of beryllium, the next electron would go into the 2p energy level. An electron added to neon would go into the 3s energy level.
- Although the next electron added to nitrogen would be added to a 2p level, it would have to be paired in the same region of space as an electron that was already there. The pairing energy in this case must be enough to offset the attraction of the electron to the nucleus.
Exercise 1.6.16
Perhaps the simplest explanation is this: The addition of 14 extra protons between lanthanum and hafnium, compared to between yttrium and zirconium, results in a considerable contraction of the atom. The electrons are pulled inward by the added positive charge in the nucleus. Thus, elements in the third row of the transition metal block are not as large as might otherwise be expected, and in some cases are even smaller than their precedent elements.
Note that this is not the only explanation. Relativistic effects are also believed to play a role in the behavior of these massive elements. Einstein's theory of relativity states that objects get heavier the faster they move. The velocity of an electron can be shown to increase with the charge in the nucleus of the atom. Thus, atoms that have very high atomic numbers have very, very fast electrons, and consequently very heavy ones. These heavy electrons sink toward the heavy nucleus, and the atom shrinks further.


