1.6: The Periodic Table and Periodic Trends
- Page ID
- 189610
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The aufbau process is a set of rules that allows us to predict the electronic configuration of an atom if we know how many electrons there are in the atom. If the periodic table is used as a tool, this process is pretty easy.
For atoms found in the first two columns of the periodic table (figure \(\PageIndex{1}\)), the configuration is a closed shell of core electrons, plus s electrons in a new shell. For example, potassium has a configuration [Ar]4s1. These atoms are often called the alkali and alkaline earth elements. Alkali elements, from the first column, have a configuration ending in s1; alkaline earth elements, from the second column, have configurations ending in s2. Together, these elements are often called the s-block elements, because their valence electrons are s electrons. Remember, the valence electrons are the ones beyond the noble gas core. In the case of potassium, they are the ones beyond [Ar].
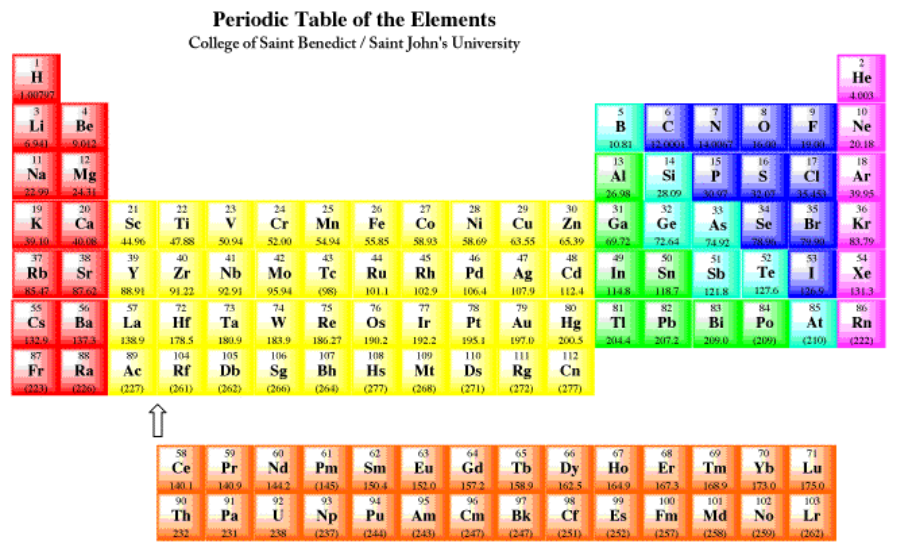
The first two and the last six columns of the periodic table are called the main group elements. Alternatively, they are sometimes called the s-block and p-block elements, respectively. For example, phosphorus has a configuration, [Ne]4s24px1py1pz1, or simply [Ne]4s24p3.
The middle block of the periodic table consists of the transition metals or the d-block elements. For example, scandium has configuration [Ne]4s23d1.
The final two rows of the periodic table are the lanthanides and actinides. Collectively, they are called the f-block elements. Samarium, for example, is [Xe]6s24f6. These elements could really be inserted at the left-hand side of the d-block in the appropriate rows. Notice that lanthanum, element 57, is followed by hafnium, element 72, in the table. The element that really occurs next is element 58, cerium, and it is shown in the lanthanide row down below. The f-block elements are usually shown below in order to save space.
Really, the periodic table should look like this:
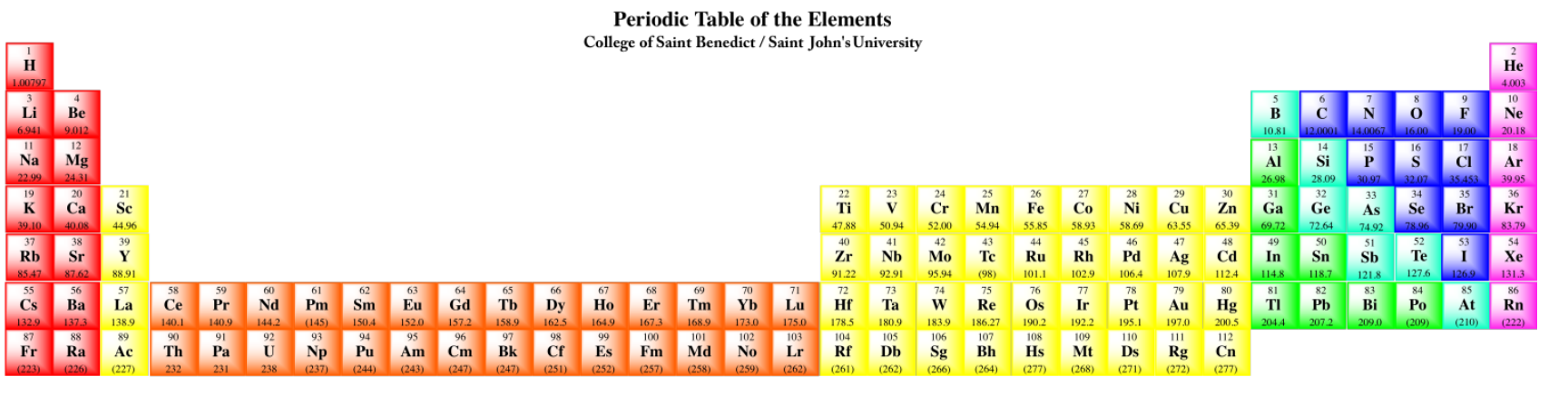
- The periodic table is divided into columns of atoms with similar electron configurations.
- Atoms with similar electron configurations have similar properties.
Chemical reactions depend on the movement of electrons. In a reaction, one atom may accept electrons from another atom. One atom may donate electrons to another atoms. The valence electrons are the outermost electrons in an atom; they are closest to the surface of an atom. That fact makes the valence electrons more likely to interact with other atoms. The valence are also the highest-energy electrons in an atom, and most likely to participate in a reaction.
For these reasons, atoms with similar electron configurations generally behave in similar ways. The repeating properties in each row of the periodic table, as observed by Mendeleev and others, reflect the repeating electron configurations in subsequent rows. The periodic table organizes atoms with similar configurations and properties together in columns.
For the following elements, suggest two other elements that would have similar properties.
a) zinc, Zn b) calcium, Ca c) oxygen, O d) chlorine, Cl e) chromium, Cr
- Answer a:
-
Zn: Cd, Hg
- Answer b:
-
Ca: Mg, Ba
- Answer c:
-
O: S, Se
- Answer d:
-
Cl: F, Br
- Answer e:
-
Cr: Mo, W
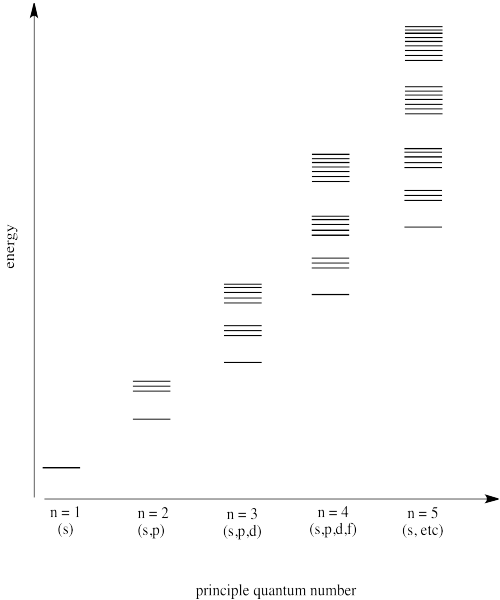
Make a diagram showing the energy levels of different orbitals, arranged by principal quantum number.
Answer
"Periodic trends" refer to the way in which physical properties of atoms change across the periodic table. One of the most commonly used periodic trends in chemistry is electronegativity. Electronegativity is closely connected to the basic idea of chemical reactions: the transfer of an electron from one neutral atom to another. It refers to how strongly an atom attracts electrons from other atoms.
- Electronegativity is a measure of an atom's ability to draw electrons towards itself, or the ability of the nucleus to hold electrons tightly.
There are many scales of electronegativity, based on different physical measurements. Usually, electronegativity is set to an approximately 4-point scale. Atoms with electronegativity of around 4 draw electrons very strongly toward themselves. Atoms with electronegativity of 1 (or lower) only weakly draw electrons toward themselves.
The following data use the Allen scale of electronegativity. The Allen scale uses spectroscopic measurements to estimate the energy of valence electrons in an atom. From these values, the relative attraction of the atom for its valence electrons is placed on a 4 point scale (approximately).
Table \(\PageIndex{1}\): The Allen electronegativity values of the second-row elements.
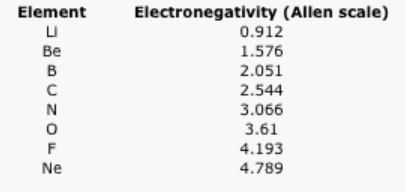
Some electronegativity scales do not have values for the noble gases, because they are based on experimental measurements of compounds, and noble gases do not commonly form compounds with other elements. Instead, they exist as single atoms. The Allen scale just depends on the ability of an atom to interact with light, which is something even noble gases can do. As a result, noble gases are also given electronegativity values on this scale. However, on many scales, fluorine would be the most electronegative atom here. As a result, fluorine is usually thought of as the most electronegative element.
Often it is useful to plot data on a graph. That way, we can get a better look at the relationship. For example, a quick glance at Figure AT5.2. shows that there is a smooth increase in electronegativity as we move across a row in the periodic table.
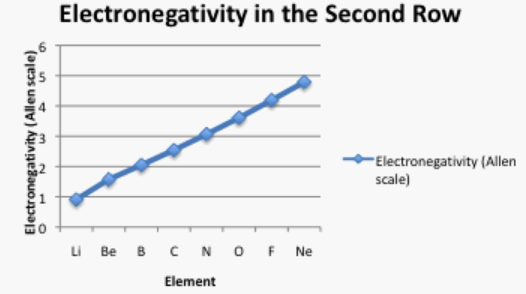
Take a look at the graph in figure \(\PageIndex{3}\). Can you explain why the electronegativity increases as atomic number increases?.
- Answer
-
The atomic number is the number of protons in the nucleus. For two atoms in the same row of the periodic table, the outermost electrons are roughly the same distance away from the nucelus. The more positive protons there are in the nucleus, the more tighly held are the electrons.
Suppose you need an electron. You have a boron atom and an oxygen atom. You try to take an electron away from one. Use Figure \(\PageIndex{3}\). to predict which atom will give up the electron more easily.
- Answer
-
Take it from the boron. The oxygen atom is holding its electrons much more tightly.
Suppose you have an electron. You are able to send it into a vessel that contains a carbon atom and a fluorine atom. Use Figure \(\PageIndex{3}\). to predict which atom is more likely to take the electron.
- Answer
-
According to the drawing, the neon would take the electron, because of all the atoms depicted in the graph, neon attracts electrons most strongly. However, there is a complication. Although neon strongly attracts its own electrons, it can't accommodate an extra electron as easily as could fluorine, the next-best candidate. In "Lewis" terms, neon has a "full octet". In quantum terms, an additional electron would have a higher principle quantum number and be placed in the next "shell", farther from the nucleus. With spin-pairing, fluorine can accept another electron into its valence shell.
A covalent chemical bond is a pair of electrons shared between two atoms. Suppose you have a carbon-oxygen bond. Will the electrons be shared evenly between the two atoms, or will one atom pull the electrons more tightly towards itself? Use Figure \(\PageIndex{3}\). to make your prediction.
- Answer
-
The oxygen would pull the electrons in the bond more tightly to itself.
What is happening as we move across a row in the periodic table? Why does electronegativity increase?
Keep in mind that the only difference from one element to the next is the number of protons in the nucleus. The number of protons is called the atomic number. If you know the number of protons you have, then you know what atoms you have. Electronegativity may have something to do with the number of protons in the nucleus. In fact, it should. The more protons there are in the nucleus, the more strongly electrons should be attracted to it. Each additional proton should add more electrostatic attraction for an electron. Fluorine, with nine protons, should attract electrons much more strongly than lithium, which has only three protons.
- Moving across a row of the periodic table, as protons are added to the nucleus, electrons are held more tightly.
- Electronegativity increases across a row in the periodic table.
It seems like that effect should be offset by the increasing number of electrons in the atom. Each time a proton is added, so is an electron. That electron should repel other electrons in the atom, cancelling out the effect of more protons in the nucleus.
However, the structure of the atom minimizes electron-electron repulsion a little bit. Remember that all the protons are in one place, the nucleus. All the electrons are a relatively great distance from the nucleus, in many different directions. Chances are, an additional electron is much farther away; it may be twice as far away as an additional proton in the nucleus. It may be all the way on the other side of the atom. Because electrons are spread out in the atom, and the distances between them is pretty large, the repulsive effect is a little smaller than the attractive effect of additional protons.
We can see that this trend is generally true across the periodic table, with a few exceptions here and there.
What happens as we move down a column in the periodic table? Table \(\PageIndex{2}\). shows the Allen electronegativities of the alkali metals. These elements are also called the Group 1 elements or the Group IA elements. The "Group 1" designation is used because they are the first column or group in the periodic table. The data are also presented in Figure \(\PageIndex{5}\).
Table \(\PageIndex{2}\): The Allen electronegativity values of the alkali elements.
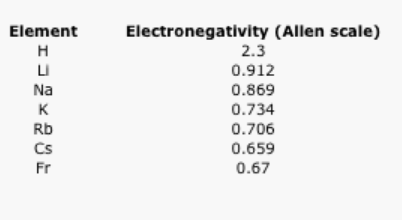
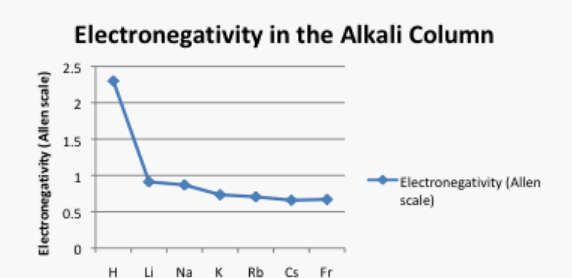
There is a different trend here. In this case, lithium (atomic number 3) has more protons than hydrogen (atomic number 1). However, hydrogen is a lot more electronegative than lithium. Francium, with 87 protons in its nucleus, is the least electronegative alkali element.
Take a look at the graph in Figure \(\PageIndex{5}\). Can you explain why the electronegativity decreases as atomic number increases, going down this column?
- Answer
-
Moving from one row to the next in the periodic table signifies that the outermost electron is in a shell farther from the nucleus. Those outermost electrons are less tightly held if they are farther from the nucleus.
An ionic chemical bond is a pair of ions attracted by their opposite charges. A cation is a positively charged ion; it may be an atom that has lost an electron. An anion is a negatively charged ion; it may be an atom that has gained an extra electron. Ions can form by moving an electron from one atom to another.
- Suppose you have an ionic cesium-fluorine bond. Which ion is the cesium and which is the fluorine? Use Figure \(\PageIndex{3}\) and Figure \(\PageIndex{5}\). to make your prediction. Cesium is Cs.
- Suppose you have an ionic sodium-oxygen bond. Which ion is the sodium and which is the oxygen? Use Figure \(\PageIndex{3}\) and Figure \(\PageIndex{5}\). to make your prediction. Sodium is Na (from the Latin, natrium).
- Suppose you have an ionic potassium-hydrogen bond. Which ion is the potassium and which is the hydrogen? Use Figure \(\PageIndex{3}\) and Figure \(\PageIndex{5}\). to make your prediction. Potassium is K (from the Latin, kalium).
- Answer a:
-
Cs+ F-
- Answer b:
-
Na+ O-
- Answer c:
-
K+ H-
Periodic Trends and Atomic Radius
The biggest difference between two atoms in the same group (column) in the periodic table is the principal quantum number. Remember, that corresponds to the "valence shell". Think of electrons as forming layers around the nucleus. Electrons with principal quantum number one form a first layer. Those with principal quantum number 2 form a second layer, and so on. Each layer is further away from the nucleus. Remember, electrostatic attraction gets weaker as charges get further away from each other. As electrons get further from the nucleus, they are less tightly held.
- Moving down a column in the periodic table, valence electrons are held less tightly because they get further from the nucleus.
- Electronegativity decreases as we move down a column in the periodic table.
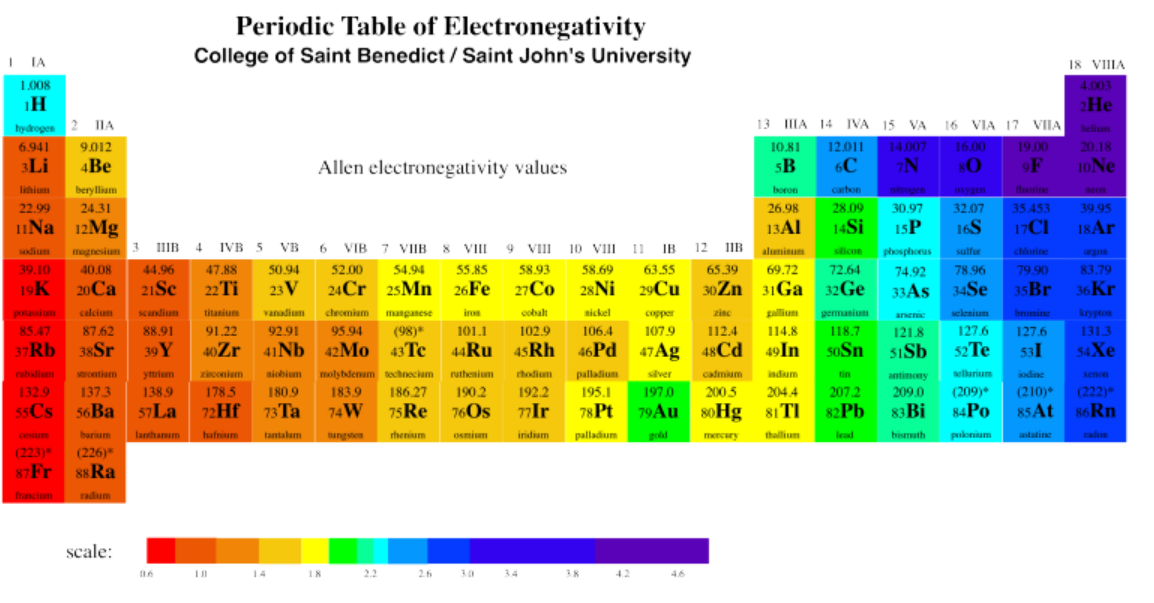
We can see this general size trend in the following periodic table. This table presents covalent radii, which are related to the sizes of the atoms (although not exactly the same; data on atomic radii are not available for all atoms, however).
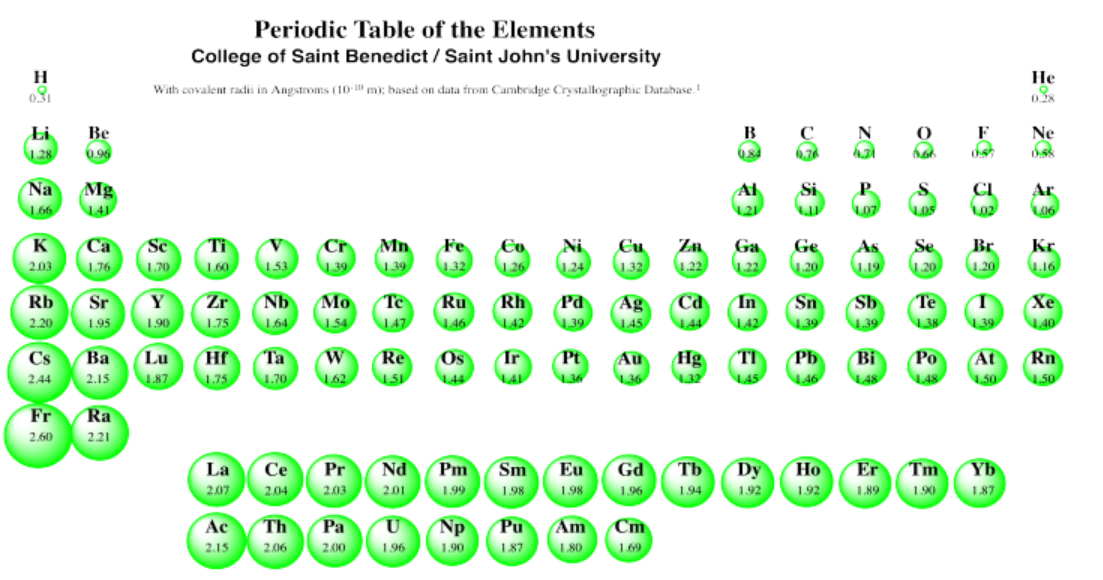
We can clearly see the expanding radii of atoms if we look at Group 1, the first column; these elements are called the alkali metals. Hydrogen, at the top, is very small. Lithium is much bigger. Sodium is much bigger than lithium, however, and potassium is much bigger than sodium. And so on: francium is bigger than cesium, which is bigger than rubidium, which is bigger than potassium.
Each time an electron is added to an orbital that is significantly farther from the nucleus, of course it is going to result in a bigger atom. Remember, the atom is mostly empty space, and its size is described by the outermost reaches of its electrons. So when we go to the next principal quantum number -- that is, to the next row in the periodic table, from the first row to the second row, for example -- the next electron is much further away from the nucleus. It has to be that way, because electrons repel each other. They can't all be equally close to the nucleus, because there would be too much repulsion. Instead, they form these layers, and when the first layer is so full that there would be too much repulsion if anothe relectron were added, we start the next layer.
Of course, the very first layer is very, very small. There just isn't that much room so close to the nucleus. For the first row, only two electrons are allowed. Then they have to start the next layer. For the second row, eight electrons are allowed; that's the origin of something called the "octet rule" (think "octopus") for common compounds, which you'll see later on. Eventually we get to eighteen electrons in a shell, then thirty two, as the shells get bigger and bigger like layers of an onion, or like nested Russian dolls.
There is another important trend if you look carefully. As you move from left to right across the periodic table, from one group to the next, the atoms get bigger. That does not make any sense, does it? If we are adding more electrons, why would the atom get smaller?
The key thing is, not only are we adding more electrons, but we are also adding more protons in the nucleus. The new electrons we are adding are all roughly equidistant from the nucleus; they are all equally close to the protons. So as the charge on the nucleus gets bigger, those electrons are all more strongly attracted to the center. The atom shrinks.
Eventually, we get to the point at which we couldn't possibly add more electrons; the radius has shrunk so much that repulsion would become too great if we added one more electron. Then we just start another row. Just before that point, however, we hit a sweet spot: the point at which the attraction between the nucleus and the outermost electrons is so strong, and the electrons are held so tightly, that the atom becomes very, very stable. This last column in the table contains the noble gases, which are particularly stable and unreactive.
Why does electronegativity fall so sharply between hydrogen and lithium, and much more subtly between lithium and sodium?
- Answer
-
The attraction for an electron falls off with 1/r2. As the value of r gets larger and larger, the quantity 1/r2 will begin to approach a limit (of zero). As a result, the difference between two successive values of 1/r2 in a series gets smaller and smaller.
Which atom, in the following pairs, is more electronegative?
- magnesium, Mg, or calcium, Ca
- lead, Pb, or tin, Sn
- silver, Ag, or antimony, Sb
- gallium, Ga, or arsenic, As
- tungsten, W, or copper, Cu
- thallium, Tl, or sulfur, S
- Answer a:
-
Mg > Ca
- Answer b:
-
Sn > Pb
- Answer c:
-
Sb > Ag
- Answer d:
-
As > Ga
- Answer e:
-
Cu > W
- Answer f:
-
S > Tl
Electron ionization is the energy that must be added in order to pull an electron away from an atom.
- Why do you think energy has to be used to pull an electron away from an atom? What is holding the electron there?
- Explain the general trend in ionization energies seen in the following table (a larger value means more energy must be added to remove a first electron from the atom).
- Answer a:
-
The electron is held by its attraction to the nucleus.
- Answer b:
-
As the number of protons in the nucleus increases, the electron becomes more tightly held, and harder to remove.
The relationship is similar to the one seen for electronegativity, and not coincidentally. Electronegativity can be calculated in a number of ways, but one of those ways uses the ionization energy as a factor.
Electronegativity is a calculated value, whereas ionization energy is an experimentally determined one. This difference brings up an important philosophical distinction. To a beginning student, an experimental value seems faulty, whereas a calculated value sounds good. To an experienced chemist, an experimental value is verifiable; it is real. In contrast, a calculated value is enhanced in prestige only if it can be shown to agree with experiment.
Table \(\PageIndex{3}\): The ionization energies of the second row elements.
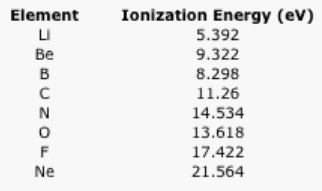
Sometimes a plot of the data can be revealing. Ionization energies do not follow a smooth trend. Explain why it is a little easier to remove an electron from boron and oxygen than expected. (Electron configurations may be helpful here.)
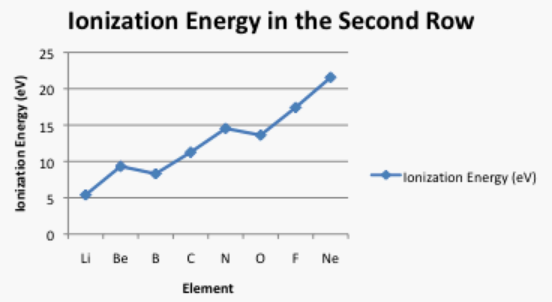
Figure \(\PageIndex{7}\): Plot of the ionization energies of the second row elements.AnswerIn order to escape the atom, an electron must gain energy. As shown in problem Exercise \(\PageIndex{2}\), the 2p energy level is higher than the 2s energy level. That means a 2p electron already has more energy than a 2s electron. The 2p electron will not need as much additional energy in order to escape from the atom. As a result, boron's ionization energy is a little lower than beryllium's.The additional protons in the nucleus of carbon and nitrogen more than make up for that effect.In the case of oxygen, the next electron is just added to a 2p level, but in this case it must be paired with another electron in the same region of space (in the same "orbital"). The repulsion between these electrons, or "pairing energy", slightly destabilizes the oxygen, so less energy will be needed to remove an electron.Once again, the continued addition of extra protons eventually compensates for this pairing effect.
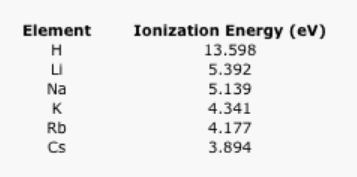
Explain the trend in the following data on ionization energy.
Table \(\PageIndex{4}\): The ionization energies of the alkali elements.
- Answer
AnswerAs with electronegativity, ionization energy decreases as the distance to the nucleus increases because of the 1/r2 relationship in Coulomb's Law.
Electron affinity is the energy released when a free electron is picked up by an atom.
- Why would energy be released when a free electron is taken by an atom?
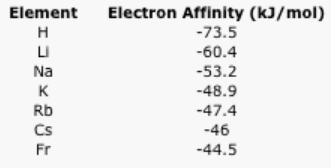
- Explain the general trend in the following electron affinity data.
Answer a:- Answer b:
Table \(\PageIndex{5}\): The electron affinities of the alkali elements.Answer a:Energy is released because of the attraction of the free electron to a nucleus. The electron moves to lower energy as it becomes stabilized by its interaction with the nucleus.Answer b:The electron can get much closer to the hydrogen nucleus thanto the lithium nucleus, and so on. More energy will be released owing to the strong interaction between the electron and the hydrogen nucleus compared to the interaction between the electron and the lithium (or sodium or potassium...) nucleus.
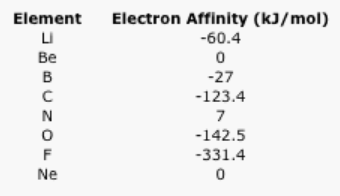
- Explain a general trend in the following electron affinity data.
- There are several exceptions to the general trend. Why do beryllium and neon have such low electron affinities (almost zero)?
- Nitrogen also has an electron affinity that is close to zero. Why?
- Answer a:
- Answer b:
- Answer c:
Answer a:The data zig-zags, but broadly speaking there is an increase in electron affinity as the number of protons in the nucleus increases.Answer b:Beryllium and neon have zero electron affinity because the next electron in each case would be added to a higher energy level. In the case of beryllium, the next electron would go into the 2p energy level. An electron added to neon would go into the 3s energy level.Answer c:Although the next electron added to nitrogen would be added to a 2p level, it would have to be paired in the same region of space as an electron that was already there. The pairing energy in this case must be enough to offset the attraction of the electron to the nucleus.
Usually, elements become bigger as we go down a column in the periodic table. However, in a phenomenon called "the lanthanide contraction", some elements are actually smaller than the ones in the row above them. Specifically, osmium, iridium, platinum, gold, and mercury are smaller than their relatives, ruthenium, rhodium, palladium, silver, and cadmium, respectively.
Use the periodic table in Figure \(\PageIndex{2}\). to offer a possible explanation for this phenomenon.
- Answer
-
Perhaps the simplest explanation is this: The addition of 14 extra protons between lanthanum and hafnium, compared to between yttrium and zirconium, results in a considerable contraction of the atom. The electrons are pulled inward by the added positive charge in the nucleus. Thus, elements in the third row of the transition metal block are not as large as might otherwise be expected, and in some cases are even smaller than their precedent elements.
Note that this is not the only explanation. Relativistic effects are also believed to play a role in the behaviour of these massive elements. Einstein's theory of relativity states that objects get heavier the faster they move. The velocity of an electron can be shown to increase with the charge in the nucleus of the atom. Thus, atoms that have very high atomic numbers have very, very fast electrons, and consequently very heavy ones. These heavy electrons sink toward the heavy nucleus, and the atom shrinks further.


