14.5: Biorefineries and Biomass Utilization
- Page ID
- 285385
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Just as crude oil is a complex mixture of hydrocarbons and other organic compounds that must be run through a petroleum refinery to separate and chemically modify the materials in it to produce fuel and petrochemical feedstocks, chemicals from biological sources are usually complex mixtures that require refining and chemical processing to provide needed feedstocks. The separation and processing of biomaterials from plants or from bacteria cultured in digesters is accomplished in a biorefinery the output of which consists of organic chemicals and fuels. In a biorefinery the various products that can be obtained from biomass are separated and subjected to chemical treatment, generally with the objective of reducing the chemically bound oxygen contents of the organic liquids. Although the large number of compounds generated from biomass poses a challenge for separations, it also provides the opportunity to produce smaller quantities of high-value chemicals.
Figure 14.3 illustrates a biorefinery showing four ways in which feedstocks may be obtained from biomass. The simplest and least energy-consumptive of these is extraction in which an organic solvent or, in more sophisticated operations, supercritical carbon dioxide is used to dissolve materials from biomass. This approach is widely used to extract oils from some kinds of oil seeds. Terpene hydrocarbons can be extracted from pinewood and other terpene-producing plants. Some of the greatest potential is to extract hydrocarbons and other oils from algae that produce these materials. Single-cell algae are particularly efficient photosynthesizers and the potential is high to genetically engineer strains that can produce specific classes of extractable organics.
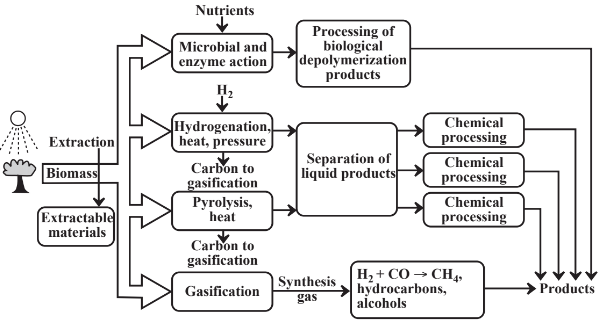
Other than by extraction, the pathway to useful chemicals from biomass involves breaking down the complex biomass polymers. One way in which this is done is with microbial action or the action of isolated enzymes, for example, to produce glucose sugar from starch or cellulose. This step may require addition of nutrients including nitrogen, phosphorus, and potassium to enable microorganisms to grow. The glucose and other monomers isolated by enzymatic action can be subjected to additional processing, the most common example of which is fermentation of glucose to alcohol.
Hydrogenation of biomass involves reaction with elemental H2 under high pressure and at elevated temperatures. This approach can be used with hydrogen generated relatively inexpensively by electrolysis of water employing renewable sources of electricity, especially from wind power. Direct hydrogenation of biomass produces a wide variety of organics including oxygenated compounds, some of which have direct uses and others of which may be chemically modified to give desired product.
Pyrolysis involves heating biomass externally or with a hot gas stream to evolve liquid and gas products. An external heat source including even solar energy focussed and concentrated on a reactor may be employed. As with hydrogenation, pyrolysis generates a variety of products including oxygenated compounds. It also produces large amounts of residual carbon, which can be used directly as fuel or gasified with steam and oxygen to produce synthesis gas.
Gasification, which is discussed in more detail in Chapter 15, Section 15.6, involves the reaction of hot carbon with steam,
\[\ce{H2O + C \rightarrow H + CO2}\]
yielding a synthesis gas mixture of H2 and CO. The carbon is usually heated by partial combustion with O2 and the CO in the synthesis gas is reacted with steam to increase the ratio of H2 to CO. Biomass can be gasified directly by reaction with a minimal amount of O2. Since biomass has the approximate empirical formula of {CH2O} the “water” required for gasification is largely in the biomass, itself. Such direct gasification of biomass also produces large quantities of organics that are processed downstream in the biorefinery.
An important consideration in biorefineries is the use of catalysts. Insofar as possible biorefineries should use heterogeneous catalysts that do not get into the product and enzyme catalysts that operate at moderate temperatures.


