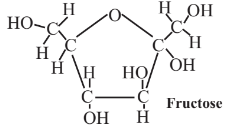
14.6: Monosaccharide Feedstocks - Glucose, Fructose, and Xylose
- Page ID
- 285386
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The monosaccharides such as glucose and fructose, as well as xylose, the monomer of hemicellulose, which makes up almost 1/3 of typical plant biomass,
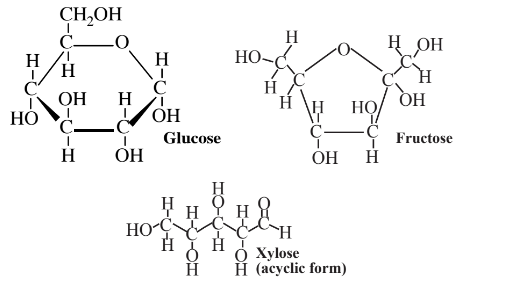
are produced in abundance by plants. These compounds are excellent platforms for a number of different organic syntheses. As partially oxidized materials, they are particularly advantageous where a partially oxidized product is made, as is often the case in organic synthesis. Monosaccharides contain hydroxyl groups (-OH) around the molecule, which act as convenient sites for the attachment of various functionalities. Glucose is metabolized by essentially all organisms, so it serves as an excellent starting point for biosynthesis reactions using enzymes, and it and many of its products are biodegradable, adding to their environmental acceptability.
Glucose can be obtained by enzyme-catalyzed processes from other sugars, including sucrose and fructose. A large fraction of the glucose that is now used is obtained from the enzymatic hydrolysis of cornstarch. It is also possible to obtain glucose by the enzymatic hydrolysis of cellulose, although it has not proven economically practical to do so on an industrial scale because of the refractory nature of the cellulose polymer. Nevertheless, the enormous quantities of cellulose available in wood and other biomass sources make glucose from cellulose an attractive prospect. The greatest use of glucose and fructose (which is readily converted to glucose by enzymes) for synthesis is by fermentation with yeasts to produce ethanol,
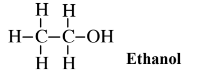
an alcohol widely used as a gasoline additive, solvent, and chemical feedstock. A byproduct of this fermentation process is carbon dioxide, the potential of which in green chemical applications as a supercritical fluid solvent are discussed in Section 13.14.
Glucose is widely used as a starting material for the biological synthesis of a number of different biochemical compounds. These include ascorbic acid, citric acid, and lactic acid. Several amino acids used as nutritional supplements, including lysine, phenylalanine, threonine, and tryptophan, are biochemically synthesized starting with glucose. The vitamins folic acid,ubiquinone, and enterochelin are also made biochemically from glucose.
In addition to the predominantly biochemical applications of glucose mentioned above, monosaccharides can be used to make feedstocks for chemical manufacture. The possibilities for so doing are now greatly increased by the availability of genetically engineered microorganisms that can be made to express genes for the biosynthesis of a number of products. Sophisticated genetic engineering is required to make chemical feedstocks because these are materials not ordinarily produced biologically.
A study by the U.S. Department of Energy Pacific Northwest Regional Laboratory has identified “top twelve value added chemicals” that can be made enzymatically from monosaccharides, especially glucose and fructose.1 Listed in Table 14.1, these chemicals could form the main feedstocks for future biorefineries that would generate an abundance of products currently made largely from petrochemicals. Several specific syntheses of commercially valuable chemicals are discussed below.
As an example of the potential of glucose for making important feedstocks, consider the synthesis from glucose of adipic acid,
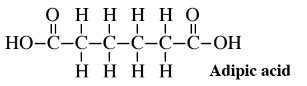
a feedstock consumed in large quantities to make nylon. The conventional synthesis of this compound starts with benzene, a volatile, flammable hydrocarbon that is believed to cause leukemia in humans. The synthesis involves several steps using catalysts at high pressure and corrosive oxidant nitric acid, which releases air pollutant nitrous oxide, N2O. The first step is the addition to benzene over a Ni/Al2O3 catalyst at a pressure 25 to 50 times atmospheric pressure of explosive hydrogen gas, H2,

to produce cyclohexane, which is then subjected to oxidation in air at 9 atm pressure over a cobalt catalyst
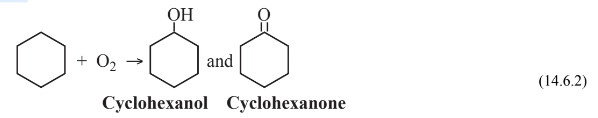
to produce a mixture of cyclohexanol, a cyclic alcohol, and cyclohexanone, a cyclic ketone. This mixture is then reacted with oxidizing, corrosive, 60% nitric acid over a Ni/Al2O3catalyst at 25–50 atm pressure to give the adipic acid feedstock:
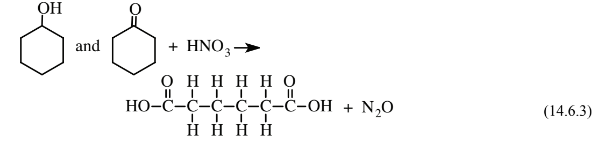
Table 14.1. Top Twelve Chemical Feedstock That Can be Made Enzymatically from Monosaccharide
| Name and structural formula | Examples of Products |
| Four carbon 1,4-diacids | Tetrahydrofuran  |
Succinic acid  |
|
Fumaric acid 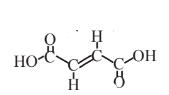 |
|
Malic acid 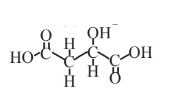 |
|
2,5-Furandicarboxylic acid  |
2,5-Bis(aminomethyl)-tetrahydrofuran 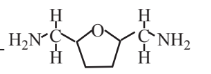 |
3-Hydroxypropionic acid 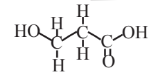 |
Methyl acrylate 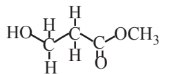 |
Aspartic acid 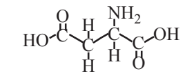 |
Aspartame (artificial sweetener) 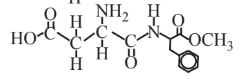 |
Glucaric acid 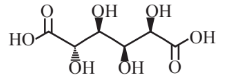 |
5-Hydroxymethyl-furfural 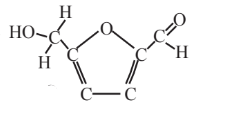 |
Glutamic acid 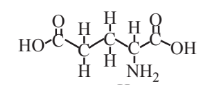 |
Proline 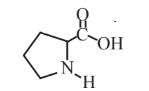 |
Itaconic acid 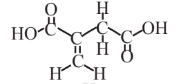 |
3-Methylpyrrolidine 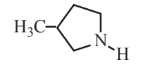 |
Levulinic acid 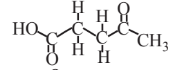 |
Acrylic acid 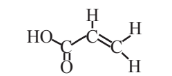 |
3-Hydroxybutyrolactone 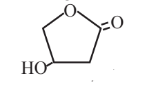 |
Epoxylactone 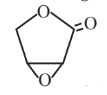 |
Glycerol 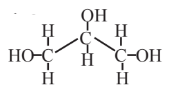 |
Propylene glycol 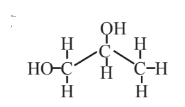 |
Sorbitol 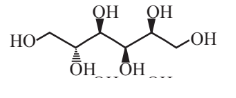 |
Sorbitol itself has numerous uses in foods(as a low-calorie sweetener) and in cosmetics |
Xylitol 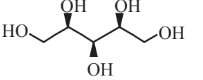 |
Ethylene glycol 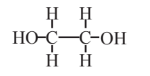 |
Throughout the synthesis process, elevated temperatures of approximately 250 ̊C are employed. The N2O released by the synthesis of adipic acid in the manufacture of nylon accounts for a significant fraction of worldwide N2O releases. The potential dangers and environmental problems with this synthesis are obvious.
As an alternative to the chemical synthesis of adipic acid described above, a biological synthesis using genetically modified Escherichia coli bacteria and a simple hydrogenation reaction has been devised. The bacteria convert glucose to cis,cis-muconic acid:
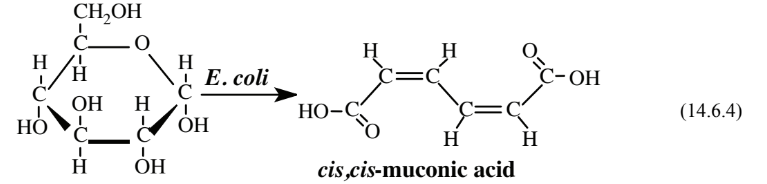
The muconic acid is then treated under relatively mild conditions with H2 under 3 atm pressure over a platinum catalyst to give adipic acid.
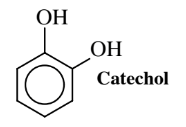
Another organic chemical that potentially can be produced by the action of transgenic microorganisms on glucose is catechol, used as a feedstock to make flavors, pharmaceuticals, carbofuran pesticide, and other chemicals. About 20 million kilograms per year worldwide of this compound are now manufactured chemically starting with propylene and carcinogenic benzene, both derived from depleting petroleum sources. Toxic phenol is generated as an intermediate, and it is oxidized to catechol with 70% hydrogen peroxide, which at this concentration is a violently reactive, hazardous oxidant. These steps require some rather severe conditions and stringent precautions in handling hydrogen peroxide reagent.E. coli bacteria of a genetically modified strain designated AB2834/pKD136/pKD9/069A, produce catechol from glucose and, if yields can be gotten to acceptable levels, biosynthesis could become a major source of this important chemical.
Another potentially important organic feedstock that has now been synthesized from glucose using transgenic E. coli is 3-dehydroshikimic acid:
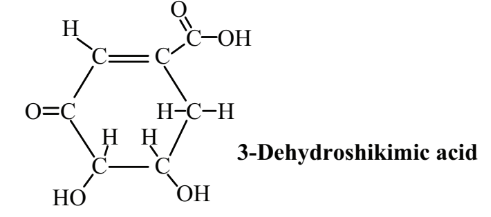
This compound is an important intermediate in the production of aromatic amino acids, gallic acid, vanillin, and other chemicals. It also has antioxidant properties. Antioxidants are organic compounds that react with oxygen-containing, reactive free radical species, such as hydroxyl radical, HO•. With their unpaired electrons (which make them free radicals), these species oxidize materials such as oils, fats, and lubricating oils and greases, causing deterioration in quality. By reacting with the free radicals, antioxidants stop their action. An abundant source of 3-dehydroshikimic acid could lead to its much wider application as an antioxidant.
The most abundant biomass feedstocks are carbohydrates. It follows that one of the most promising pathways to obtaining useful raw materials and fuels from biomass is their synthesis directly from carbohydrates. One of the more promising end products from chemical modification of carbohydrates is dimethylfuran, an oxygen-containing cyclic organic compound that has most of the desirable properties of hydrocarbons as a fuel and raw material. Compared to ethanol, dimethylfuran has a relatively low boiling temperature, has a high energy content per unit mass, does not absorb water, and exhibits combustion characteristics comparable to those of commonly used hydrocarbon fuels. Structurally, dimethylfuran with its 5-membered ring resembles the abundant monosaccharide fructose, which also has a 5-membered ring,