14.7: Cellulose
- Page ID
- 285387
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The most abundant natural material produced by organisms is cellulose synthesized biologically by the joining of glucose molecules with the loss of 1 H2O molecule for each bond formed (see Figure 14.5). This makes the chemical formula of cellulose (C6H10O5)n, where n ranges from about 1500 to 6000 or more. Most cellulose is made by plants, with total amounts exceeding 500 billion metric tons per year world-wide. Cellulose makes up the sturdy cell walls of plants. Wood is about 40% cellulose, leaf fibers about 70%, and cotton, one of the purest sources of cellulose, about 95%. Cellulose occurs in different forms and is generally associated with hemicellulose (a material also composed of carbohydrate polymers) and lignin, a biopolymer of varied composition and bonding composed largely of aromatic unit.
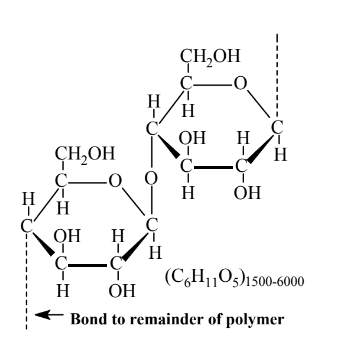
The first major step in cellulose utilization, such as extraction of cellulose fibers for making paper, consists of separating the cellulose from its matrix of lignocellulose (hemicellulose and lignin). This step has been the cause of many problems in utilizing cellulose because of the harsh chemical processing that has been employed. Lignin residues impart color to the cellulose, so wood pulp used in making paper has to be bleached with oxidants that alter the structure of the coloring agents. Bleaching used to be done almost entirely with elemental chlorine, Cl2, and salts of hypochlorite ion, ClO-. However, bleaching of biomass with these chlorine-based materials produces chlorinated organic impurities and pollutants. Therefore, ozone and hydrogen peroxide are preferred bleaching agents.
A finely divided form of cellulose called microcrystalline cellulose is produced by appropriate physical and chemical processing of cellulose. This material has many uses in foods in which it impart smoothness, stability, and a quality of thickness. Microcrystalline cellulose is also used in pharmaceutical preparations and cosmetics. Added to food, indigestible cellulose contributes bulk and retains moisture.
Chemically modified cellulose is used to make a wide variety of materials. Like the glucose that comprises it, cellulose has an abundance of -OH groups to which various other groups can be bonded to impart a variety of properties. One of the oldest synthetic fabrics, rayon, is made by treating cellulose with base and carbon disulfide, CS2, then extruding the product through fine holes to make thread. In a similar process, chemically treated cellulose is extruded through a long narrow slot to form a sheet of transparent film called cellophane.
As seen by the structural formula in Figure 14.5, each unit of the cellulose polymer has three–OH groups that are readily attached to other functional groups leading to chemically modified cellulose. One of the most common such products is cellulose acetate, an ester (see Section 6.4and Reaction 6.4.1) used primarily for apparel and home furnishings fabrics in which most of the -OH groups on cellulose are replaced by acetate groups by reaction with acetic anhydride (see below):
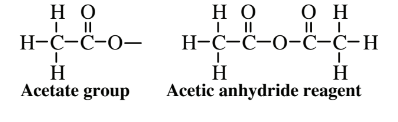
Although the cellulose feedstock for cellulose acetate synthesis is certainly a “green” material, acetic anhydride used to make the acetate is a corrosive, toxic chemical that produces poorly chealing wounds on exposed flesh. Furthermore, potentially hazardous solvents, such as dichloromethane, are used in some processes for making cellulose acetate.
Another cellulose ester that has been widely manufactured is cellulose nitrate in which the−OH groups on cellulose are replaced by −ONO2 groups by treating cellulose with a mixture of nitric acid (HNO3) and sulfuric acid (H2SO4). Cellulose nitrate makes transparent film and was used in the early days of moving pictures for movie film. However, one of the other major uses of this material is as an explosive, so cellulose nitrate can burn violently giving off highly toxic fumes of NO2 gas. In years past this characteristic has lead to several tragic fires involving human fatalities. Its use is now largely restricted to lacquer coatings, explosives and propellants. Although the cellulose raw material is green, neither the process for making cellulose nitrate involving strong acids, nor the flammable product would qualify as green.
From the discussion above, it is apparent that cellulose is an important raw material for the preparation of a number of materials. The reagents and conditions used to convert cellulose to other products are in some cases rather severe. It may be anticipated that advances in the science of transgenic organisms will result in alternative biological technologies that will enable conversion of cellulose to a variety of products under relatively mild conditions.


