14.4: Biological Sources of Chemicals
- Page ID
- 285384
In the provision of specialty and commodity chemicals and feedstocks, there are two main biological sources of materials. One of these consists of plants, which make huge quantities of cellulose and lesser quantities of other materials by photosynthesis. The other source is microorganisms, especially bacteria and yeasts.
Fermentation
Fermentation refers to the action of microorganisms on nutrients under controlled conditions to produce desired products. Fermentation for some products is anoxic (absence of O2); for other products oxic fermentation may be required. Fermentation processes have been used for thousands of years to produce alcoholic beverages, sauerkraut, vinegar, pickles, cheese, yogurt, and other foods. Ethanol, the alcohol in alcoholic beverages, is the most widely produced chemical made by fermentation. Lactic acid,
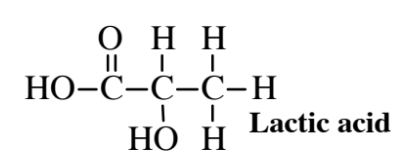
has also been produced by fermentation processes for many years. More recently, fermentation has been applied to the production of a wide variety of organic acids, antibiotics, enzymes, and vitamins.
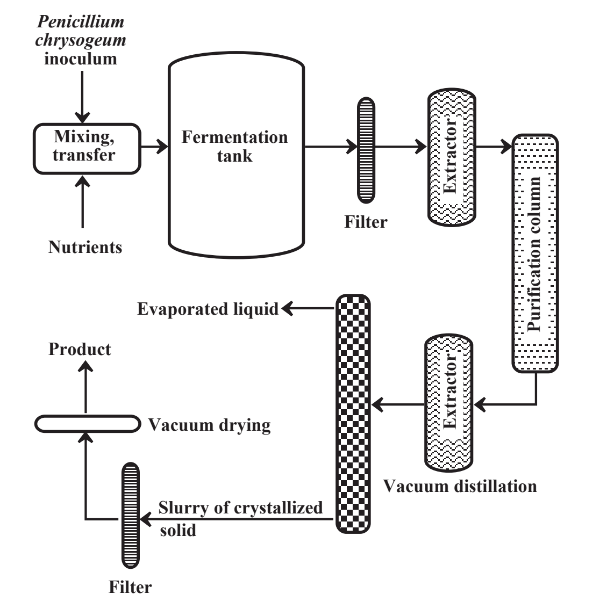
Starting in the 1940s, one of the major products of industrial fermentation has been penicillin, of which there are several forms. Figure 14.2 shows a simplified diagram of a facility for production of this life-saving antibiotic. Following penicillin, fermentation processes were developed for the production of several other significant antibiotics.
Selection of the appropriate microorganism is the most important consideration of a successful fermentation production process. The microorganisms have to have the proper nutrients, the choice of which can affect the kind and yield of the product. Sterile conditions must be maintained, and sterilization of equipment and media is accomplished by heating to 125–150 ̊C for appropriate lengths of time. Air entering the fermenter must be filtered and sterilized. The temperature of fermentation is important, with fermentation rates increasing up to an optimum temperature, after which they decrease sharply with increased temperatures as the enzymes used by the microorganisms are destroyed (denatured). This kind of temperature relationship has increased interest in the use of thermophilic microorganisms that exist at boiling water temperatures in hot springs. If such organisms can be engineered to produce desired products, the rate of product generation may increase markedly. Both the levels of oxygen (which must be excluded from anoxic processes) and pH must be controlled precisely. Modern fermentation processes use a variety of sensors to continuously monitor conditions in the fermentation tank and computerized control to accurately control all the parameters.
Fermentation is undergoing tremendous development with the use of transgenic microorganisms to which genes have been transferred to make specific kinds of substances. The most common and valuable substances made by transgenic microorganisms consist of a variety of proteins. These include proteins and smaller molecule polypeptides that are used as pharmaceuticals. The best example of such a substance is human insulin, which is now produced in large quantities by transgenic microorganisms.
Until recently, fermentation has not been widely employed to make commodity chemicals used on a large scale. An exception is the large-scale production of ethanol from the fermentation of glucose sugar by yeasts. Now mandated as a gasoline additive in the U. S. by law, huge and growing quantities of ethanol are made by fermentation of glucose derived from corn and this use is an important market for corn. It is not clear that this is a truly green technology and some authorities believe that the energy consumed and the environmental damage from more intensive cultivation of corn outweigh the benefits of using this grain to produce ethanol fuel. Advances in transgenic microbiology have increased the possibilities for using fermentation to produce a variety of chemicals and chemical feedstocks, several examples of which are discussed in this chapter.
Production of Materials by Plants
The uses of microorganisms operating in fermentation processes to generate commodity chemicals were discussed above. Plants are the other kind of organism that can be used for producing chemicals. Indeed, the nutrients used for fermentation processes come originally from plants. Fermentation is in a sense not a very efficient means of producing chemicals because of the consumption of nutrients to support the microorganisms and their reproduction and because of the generation of large quantities of byproducts. Plants, which generate their own biomass from atmospheric carbon dioxide and water are very efficient producers of materials. Wood and the cellulose extracted from it are prime examples of such materials.
In addition to their efficient production of biomass, plants offer distinct advantages in their production and harvesting. Genetics determine the materials that a plant makes, and once a crop is growing in a field, the products it is programmed for will be produced without fear of contamination by other organisms, which is always a consideration in fermentation. Plants can be grown by relatively untrained personnel using well known agricultural practices. Plant matter is generally easy to harvest in the form of grains, stalks, and leaves, which can be taken to a biorefinery (see below) to extract needed materials.
The production of feedstocks and other chemical commodities from plants has been limited by the genetic restrictions inherent to plants. Now, however, transgenic plants can be bred to produce a variety of materials directed by genes transplanted from other kinds of organisms. For example, as discussed in Section 14.10, plants have even been developed to synthesize plastics. Another limitation of the production of materials by plants has been the mixture of these materials with other matter generated by plants. The intimate mixture of useful wood cellulose with lignin, for which uses are still being sought, is a prime example of this problem. Again, transgenic technology can be expected to be helpful in developing plants that produce a relatively pure product (such as the almost pure cellulose in cotton.)
The potential of plants to produce useful products has been greatly increased by the development of hybrid plants with spectacular capacities to generate biomass by photosynthesis. Corn is one of the more productive field crops, and hybrid varieties produce large quantities of grain and plant biomass (leaves, stalks, husks and cobs commonly called corn stover). Sugar cane is noted for its ability to produce biomass, some in the form of sugar, much more in the cane stalk biomass. The sugar cane stalk residues left after extracting sugar from it (bagasse) has had relatively few uses, other than for fuel but potentially can produce large quantities of chemical feedstocks in biorefineries. One of the more exciting developments of productive hybrid plants is the hybrid poplar tree which, nourished by minimal amounts of fertilizer and watered by economical trickle irrigation systems, grows within a few years to a harvestable size for the production of wood pulp and wood for plywood. The ability of these trees to generate cellulose that can be converted to glucose means that they may serve as the basis of an entire plant-based chemicals industry. The possibility exists that they can be genetically engineered to produce other chemicals as well.


