13.13: Energizing Chemical Reactions and Process Intensification
- Page ID
- 285718
One of the most important aspects of green chemistry is the enhancement of the speed and degree of completion of chemical reactions. One of the ways that this is done is by lowering the activation energy required to enable a reaction to proceed. That is what catalysts do as discussed in the preceding section. The other way to enhance a reaction is by adding energy as discussed in this section.
The most straightforward means to add energy to a reaction is by heating the reaction mixture. On an industrial scale this is commonly accomplished with coils of tubing immersed in the reaction mixture that are heated with steam passing through the coils. Heating by passing a current of electricity through electrically-resistant coils is also a means of adding energy to a chemical system. Much of the effort in green chemistry has been devoted to finding more sophisticated ways of energizing chemical systems.
Microwaves can be used to add energy to reactions to enhance reaction rates. Microwaves are electromagnetic radiation with wavelengths of 1 cm to 1 m (frequency 30 GHz to 300 Hz). To avoid interference with microwave bands used in communication, industrial and household microwave generators commonly operate at 2.45 GHz. Microwaves are absorbed by polar molecules, such as those of water, causing rapidly repeating re-orientation of the molecules in a microwave field. The result is a high input of energy directly into substances subjected to microwaves thereby adding energy and speeding up reactions. Microwave energy can be put directly into relatively small volumes of reaction media, reducing material requirements and minimizing wastes. Microwaves can be used to enhance reactions in (1) water media, (2) polar organic solvents such as dimethylformamide, and (3) media-free reactions, such as mixed solid reactants.
Sonochemistry adds energy by subjecting a reaction medium to ultrasound energy at frequencies between 20 and 100 KHz which introduces very high energy pulses into the medium. Commonly, the ultrasound is produced by the piezoelectric effect through which crystals of substances such as ceramic-impregnated barium titanate are subjected to rapidly reversing electrical fields converting the electrical energy to sound energy with an efficiency that can reach 95%. An advantage of sonochemistry is that it can introduce high energy into microscopic regions enabling reactions to occur without appreciably heating the reaction medium.
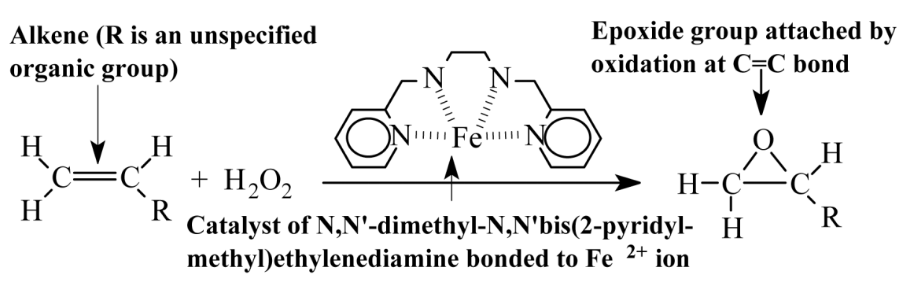
Electrochemistry by the passage of a direct current of electricity through a reaction medium can cause both reductions and oxidations to occur. Reduction, the addition of electrons, e-, occurs at the relatively negatively charged cathode, and oxidation, the loss of electrons, at the relatively positively charged anode. Electrochemical oxidation and reduction can be controlled by the electrical potentials applied, by the media in which they occur, and by the electrodes used. Because the addition of electrons to the reaction medium (reduction) and their accompanying removal (oxidation) does not add matter, electrolytic syntheses meet the goals of green chemistry. The electrolytic production of oxygen and of hydrogen, a non-polluting fuel and valuable raw material, is shown in Section 13.15 and Figure 13.10.
Photochemical reactions use the energy of photons of light or ultraviolet radiation to cause reactions to occur. The energy, E, of a photon of electromagnetic radiation of frequency,ν, is E =hν, where h is Planck’s constant. Since a photon can be absorbed directly by a molecule or a functional group on a molecule, the application of electromagnetic radiation of the appropriate energy to a reaction medium can introduce a high amount of energy into a reactant species without significantly heating the medium. Photochemical energy can be used to cause synthesis reactions to occur more efficiently and with less production of waste byproducts than nonphotochemical processes. One example is the acylation of benzoquinone with an aldehyde to produce an acyl hydroquinone, an intermediate used to make some specialty polymers:
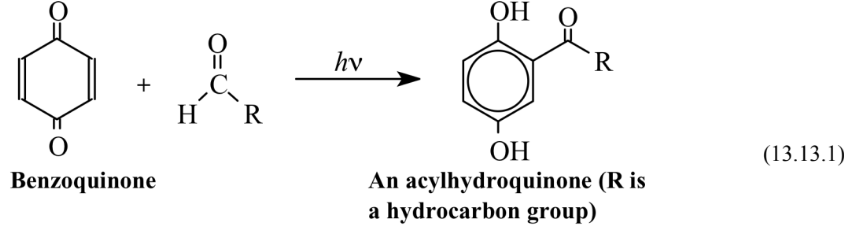
This reaction occurs with 100% atom economy. Unlike the standard Friedel-Crafts type of reaction, which utilizes the catalytic effect of Lewis acid-type acidic halides, particularly aluminum chloride, AlCl3, the photochemical process does not require catalytic substances which tend to be reactive and moisture- and air-sensitive.
A reaction participant does not have to absorb a photon directly to undergo a photochemically induced reaction. In some cases photochemically reactive species are added to the reaction mixture to absorb photons, then produce reactive excited species or free radicals that carry out additional reactions. An example of this is provided with hydrogen peroxide, which absorbs photons.
\[\ce{H2O2 + } h \nu \rightarrow \ce{ HO \cdot + HO \cdot}\]
to produce reactive hydroxyl radicals that react with a number of other species.
Process Intensification and Increased Safety with Smaller Size
Process intensification can be employed with continuous-flow reactors (Figure 13.8) used to intensify chemical processes and enable increased output of product with a smaller footprint of apparatus. This is especially the case when continuous flow is combined with heterogeneous catalysis and energy input. A big benefit to such reactors from the green chemistry viewpoint is increased safety. If something goes wrong in a large batch reactor, in the worst case an accident such as an explosion or fire with a large amount of material may occur. With a continuous-flow reactor the problem can be confined to the small volume of the reactor and the process can be shutdown immediately by stopping the inflow of the reactants.


