13.14: Solvents and Alternate Reaction Media
- Page ID
- 285719
Chemical reactions are often carried out in media, usually organic solvents or water, to provide a medium in which feedstocks and reagents can dissolve and come into close, rapid contact at the molecular level. A good solvent for chemical synthesis is one that enables facile product separation and is amenable to purification and reuse with minimum loss. Substances dissolved in a solvent are solvated by binding of the solvent to the molecules or ions of the dissolved substance, the solute. Solvation of reactants and products often plays an important role in determining the kinds and rates of reactions. Many organic feedstocks and reagents are not soluble in water or are decomposed by it, so organic solvents including hydrocarbons, chlorinated hydrocarbons, and ethers have to be used as reaction media.
Organic solvents cause several problems in chemical synthesis. Particularly because of problems associated with their containment, recovery, and reuse, organic solvents especially are major contributors to undesirably high E factors. Many of the environmental and health problems associated with making chemicals are the result of the use of organic solvents as media. Hydrocarbon solvents will burn and hydrocarbon vapors in air are explosive. Although many hydrocarbon solvents are not particularly toxic, some can cause the condition of peripheral neuropathy (damage to peripheral nerves such as those in feet and legs), and benzene is regarded as a carcinogen thought to cause leukemia. Released to the atmosphere, hydrocarbons can also participate in photochemical processes leading to the formation of photochemical smog (see Chapter 10, Section 10.11).
One approach to making chemical synthesis processes greener is to replace specific solvents with less hazardous ones. For this reason, toxic benzene solvent is replaced by toluene wherever possible. As shown by their structural formulas below, toluene has a methyl group,−CH3, that benzene does not possess. The methyl group in toluene can be acted upon by human metabolic systems to produce a harmless metabolite (hippuric acid) that is eliminated in the urine, whereas metabolic processes acting upon benzene convert it to a toxic intermediate that can react with cellular DNA.

As another example of solvent replacement, n-hexane, which can cause peripheral neuropathy in exposed individuals, can be replaced with 2,5-dimethylhexane, which does not cause this condition, for reactions where the higher boiling temperature of the latter compound is not a problem.
There is significant interest in reaction media other than organic solvents. The ultimate approach to eliminating problems with solvents in chemical synthesis is to do reactions without solvents of any kind. Some reactions can be performed in which the reactants are simply mixed together or are held on solid supports, such as clays. Microwave heating of such reaction mixtures has proven effective in providing energy to enable reactions to occur rapidly. However, in many cases that is not possible and solvents are required. Some alternative solvents are discussed below.
Water Solvent
Although many reagents are reactive with water making its use impossible, where applicable, the greenest solvent for green chemical processes is water. Water is abundant, cheap, not toxic, and does not burn. Because of its polar nature and the ability to form hydrogen bonds (see Chapter 9, Section 9.1 and Figure 9.1), water is an especially good solvent for ionic compounds — acids, bases, and salts. Water is particularly useful as one of the solvents in biphasic catalysis described in Section 13.11. Normally the catalyst is held in the water phase and the product in a water-immiscible organic solvent, which allows facile separation of the catalyst after the reaction is complete.
Because of water’s many advantages, significant efforts have been made in replacing organic solvents used for reaction media with water. Although water does not appreciably dissolve many nonpolar organic compounds, in some cases these materials may be suspended as very small colloidal particles in water, enabling close enough contact of organic materials to undergo reactions. Water is a good solvent for some of the biological materials, such as glucose, now favored as chemical feedstocks where they can be used.
Carbon Dioxide Solvent
At a high pressure above 73.8 atm (73.8 times normal atmospheric pressure at sea level) and a temperature exceeding 31.1 ̊C, carbon dioxide becomes a supercritical fluid, a relatively dense state of matter in which there is no longer a distinction between liquid and gas. A good solvent for organic compounds, supercritical carbon dioxide can be used as a reaction medium for organic chemical reactions. An advantage of supercritical carbon dioxide in this application is that its viscosity is only about 1/30 that of common liquid organic solvents, which enables reactant species to move much faster through the fluid, thus speeding the reactions that they undergo. At temperatures and pressures somewhat below those at which carbon dioxide becomes critical, it exists as separate gas and liquid phases while retaining many of the solvent properties of supercritical carbon dioxide. Under these conditions carbon dioxide is called a dense phase fluid, a term that also encompasses supercritical fluids.
Adjustment of the composition and conditions under which dense phase fluid carbon dioxide is maintained can provide significant variations in its solvent properties and adjustment of its ability to act as a reaction medium. In addition to variations in temperature and pressure, dense phase fluid carbon dioxide may be mixed with small quantities of other solvents (modifiers), such as methanol, to further vary its solvent properties.
In addition to its solvent properties, dense phase fluid carbon dioxide offers the advantage of low toxicity and low potential for environmental harm (the small amounts of greenhouse gas carbon dioxide released from its application as a solvent are negligible compared to quantities released from combustion of fossil fuels). A big advantage of dense phase fluid carbon dioxide is its volatility, meaning that it separates readily from reaction products when pressure is released. Furthermore, carbon dioxide released from a reaction mixture can be captured and recycled for the same application. Carbon dioxide can be obtained at low cost from biological fermentation processes.
Ionic Liquid Solvents
Ionic liquids present another alternative to organic solvents for use as media for chemical synthesis. Inorganic salts consisting of ions, such as NaCl composed of Na+ and Cl-ions, are normally hard, high-boiling solids. However, when one or both of the ions are composed of large charged organic molecules, as shown by the cation in the example of an ionic liquid below,
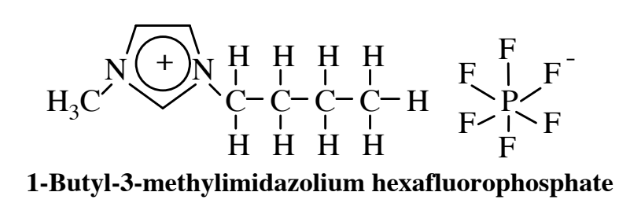
the salts can be liquids at room temperature and are called ionic liquids. These materials have the potential to act as suitable media in which substances can be dissolved and undergo reactions, and active research is underway to explore this possibility. There is an enormous variety of such ionic liquids with widely varying solvent properties because of the large number of kinds of ions that can be combined leading to almost limitless possibilities for various ionic liquids. An interesting possibility that has been tried experimentally is to use a mixed ionic liquid/supercritical carbon dioxide single fluid phase in which the reaction proceeds with a homogeneous catalyst followed by reduction of pressure that causes the supercritical carbon dioxide and ionic liquid to separate into two phases with the catalyst remaining in the ionic liquid — hence available for reuse — and the product in the supercritical carbon dioxide.


