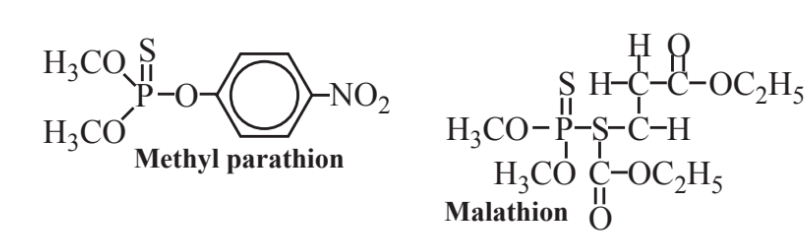6.4: Functional Groups
- Page ID
- 285310
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Numerous elements in addition to carbon and hydrogen occur in organic compounds. These are contained in functional groups, which define various classes of organic compounds. The −NH2 group in aniline and the -OH groups in phenol mentioned above are examples of functional groups. The same organic compound may contain two or more functional groups. Among the elements common in functional groups are O, N, Cl, S, and P. There is not space here to discuss all the possible functional groups and the classes of organic compounds that they define. Some important examples are given to provide an idea of the variety of organic compounds with various functional groups. Other examples are encountered later in the text
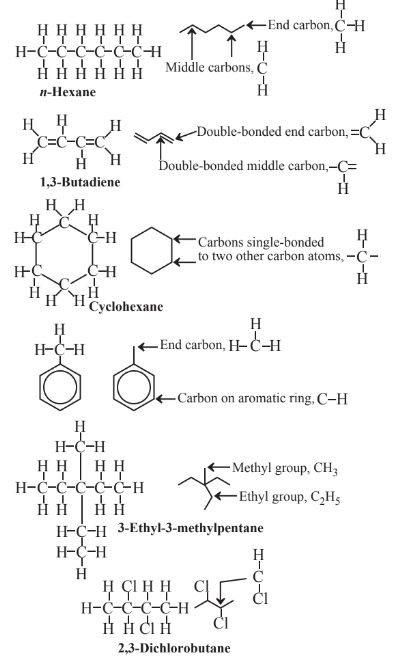
In using lines to represent organic structural formulas, the corners where lines intersect and the ends of lines represent C atoms, and each line stands for a covalent bond (2 shared electrons). It is understood that each C atom at the end of a single line has 3 H atoms attached, each C atom a the intersection of 2 lines has 2 C atoms attached, each C at the intersection of 3 lines has 1 H attached, and the intersection of 4 lines denotes a C atom with no H atoms attached. Multiple lines
Organooxygen Compounds
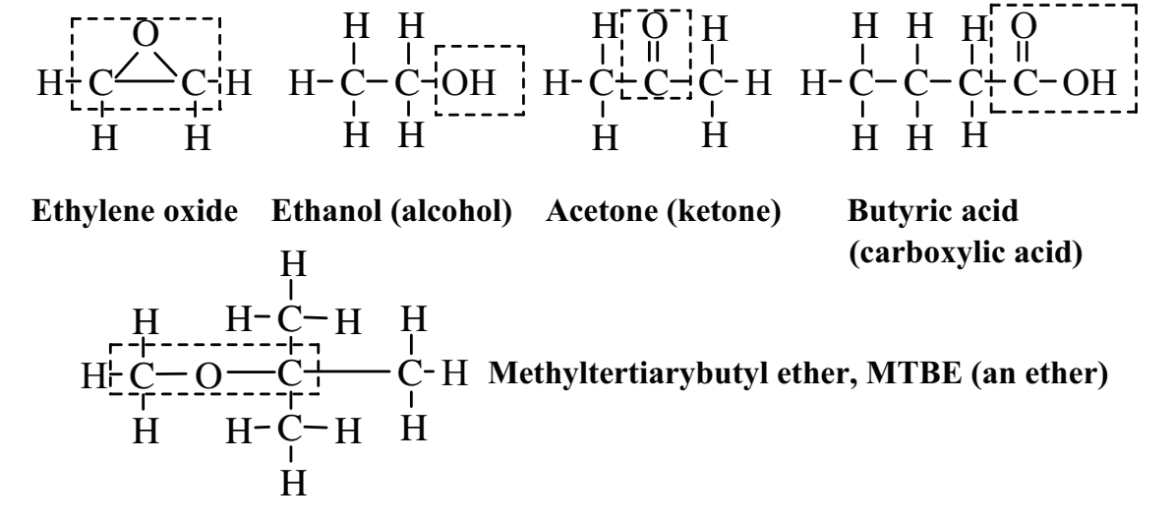
Figure 6.4 shows several important classes of organic compounds that contain oxygen. Ethylene oxide is a sweet-smelling, colorless, flammable, explosive gas. It is an epoxide characterized by an oxygen atom bridging two carbon atoms that are also bonded with each other. Ethylene oxide is toxic and is used as a sterilant and fumigant as well as a chemical intermediate. Because of the toxicity and flammability of this compound, the practice of green chemistry tries to avoid its generation and use. Ethanol, which occurs in alcoholic beverages, is an alcohol, a class of compound in which the -OH group is bonded to an alkane or alkene (attachment of the -OH group to an aromatic hydrocarbon molecule gives a phenolic compound). Acetone is a ketone, a class of compounds that has the C=O functional group in the middle of a hydrocarbon chain. Acetone is an excellent organic solvent and relatively safe. Butyric acid, which occurs in butter, is an organic carboxylic acid, all of which contain the functional group,
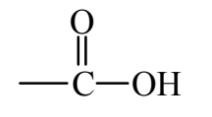
which can release the H+ion characteristic of acids. Methyltertiarybutyl ether, MTBE, is an example of an ether in which an O atom connects 2 C atoms. When highly toxic tetraethyllead was phased out of gasoline as an octane booster, MTBE was chosen as a substitute. It was subsequently found to be a particularly noxious water pollutant, and its use has been largely banned
The C=O group in the middle of an organic molecule is characteristic of ketones. When this group is located at the end of a molecule and the carbon is also bonded to H, the compound is an aldehyde. The two lowest aldehydes are formaldehyde and acetaldehyde,

of which formaldehyde is the most widely produced. Despite its many uses, formaldehyde lacks characteristics of green chemicals because it is a volatile, toxic, noxious substance. Formaldehyde tends to induce hypersensitivity (allergies) in people who inhale the vapor or whose skin is exposed to it.
The reaction of an alcohol and an organic acid,
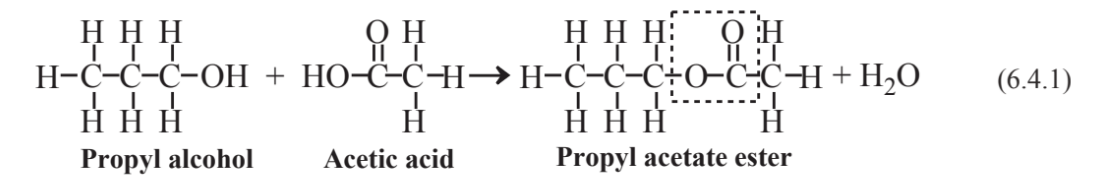
produces an important kind of organic compound called esters. The linkage characteristic of esters is outlined by the dashed box in the structure of propyl acetate above. A large number of the naturally-occurring esters made by plants are noted for their pleasant odors. Propyl acetate, for example gives pears their pleasant odor. Other fruit odors due to esters include methyl butyrate, apple; ethyl butyrate, pineapple; and methyl benzoate, ripe kiwi fruit.
Organonitrogen Compounds
Methylamine,
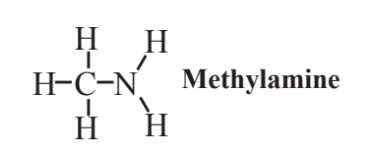
is the simplest of the amines, compounds in which an N atom is bonded to a hydrocarbon group. In an amine, the N atom may be bonded to 2 H atoms, or one or both of these H atoms may be substituted by hydrocarbon groups as well. Although it is widely used in chemical synthesis because no suitable substitutes are available, methylamine is definitely not compatible with the practice of green chemistry. That is because it is highly flammable and toxic. It is a severe irritant to skin, eyes, and mucous membranes of the respiratory tract. It has a noxious odor and is a significant contributor to the odor of rotten fish. In keeping with the reputation of amines as generally unpleasant compounds, another amine, putrescine, gives decayed flesh its characteristic odor.
Many organonitrogen compounds contain oxygen as well. One such compound is nitromethane

used in chemical synthesis and as a fuel in some race cars. As seen in the structural formula above, the nitro group, -NO2, is the functional group in this compound and related nitro compounds. Another class of organonitrogen compounds also containing oxygen consists of the nitrosamines, or N-nitroso compounds, which have figured prominently in the history of green chemistry before it was defined as such. These are compounds that have the N-N=O functional group, which are of concern because several are known carcinogens (cancer-causing agents). The most well known of these is dimethylnitrosamine shown below:
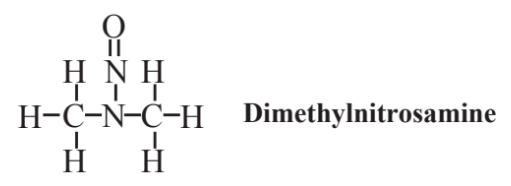
This compound used to be employed as an industrial solvent and was used in cutting oils. However, workers exposed to it suffered liver damage and developed jaundice, and the compound as well as other nitrosamines was found to be a carcinogen. A number of other nitrosamines were later found in industrial materials and as byproducts of food processing and preservation. Because of their potential as carcinogens, nitrosamines are avoided in the practice of green chemistry.
Organohalide Compounds
Organohalides exemplified by those shown in Figure 6.5 are organic compounds that contain halogens — F, Cl, Br, or I — but usually chlorine, on alkane, alkene, or aromatic molecules. Organohalides have been widely produced and distributed for a variety of applications, including industrial solvents, chemical intermediates, coolant fluids, pesticides, and other applications. They are for the most part environmentally persistent and, because of their tendency to accumulate in adipose (fat) tissue, they tend to undergo bioaccumulation and biomagnification in organisms
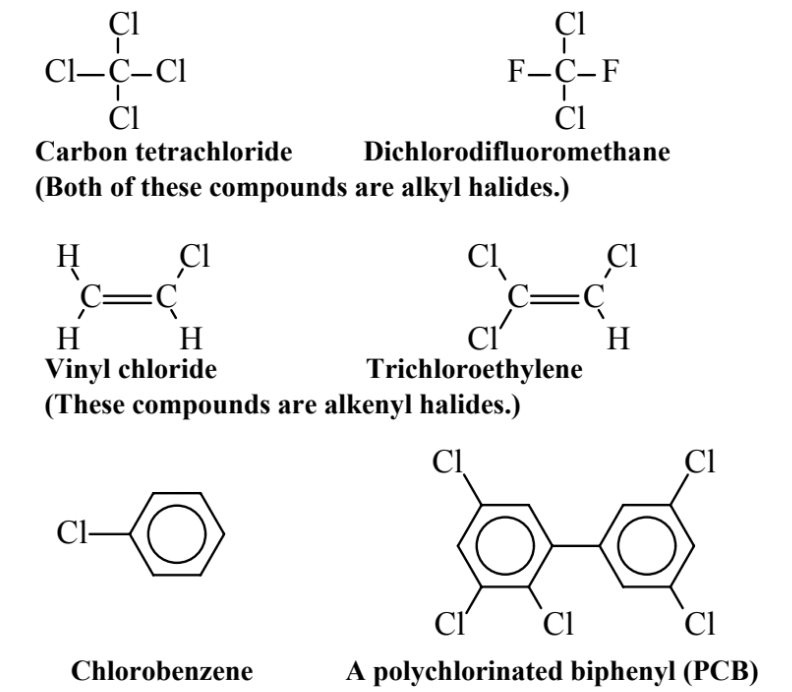
Carbon tetrachloride is produced when all four H atoms on methane, CH4, are substituted by Cl. This compound was once widely used and was even sold to the public as a solvent to remove stains and in fire extinguishers, where the heavy CCl4 vapor smothers fires. It was subsequently found to be very toxic, causing severe liver damage, and its uses are severely restricted. Dichlorodifluoromethaneis a prominent member of the chlorofluorocarbon class of compounds, popularly known as Freons. Developed as refrigerant fluids, these compounds are notably unreactive and nontoxic. However, as discussed in Chapter 10, they were found to be indestructible in the lower atmosphere, persisting to very high altitudes in the stratosphere where chlorine split from them by ultraviolet radiation destroys stratospheric ozone. So the manufacture of chlorofluorocarbons is now prohibited. Vinyl chloride, an alkene-based organohalide compound, is widely used to make polyvinylchloride polymers and pipe. Unfortunately, it is a known human carcinogen, so human exposure to it is severely limited. Trichloroethylene is an excellent organic solvent that is nonflammable. It is used as a dry cleaning solvent and for degreasing manufactured parts, and was formerly used for food extraction, particularly to decaffeinate coffee. Chlorobenzene is the simplest aromatic organochloride. In addition to its uses in making other chemicals, it serves as a solvent and as a fluid for heat transfer. It is extremely stable, and its destruction is a common test for the effectiveness of hazardous waste incinerators. The polychlorinated biphenyl(PCB) compound shown is one of 209 PCB compounds that can be formed by substituting from 1 to 10 Cl atoms onto the basic biphenyl (two-benzene-ring) carbon skeleton. These compounds are notably stable and persistent, leading to their uses in electrical equipment, particularly as coolants in transformers and in industrial capacitors, as hydraulic fluids, and other applications. Their extreme environmental persistence has led to their being banned. Sediments in New York’s Hudson River are badly contaminated with PCBs that were (at the time, legally) dumped or leaked into the river from electrical equipment manufacture from the 1950s into the 1970s.
From the discussion above, it is obvious that many organohalide compounds are definitely not green because of their persistence and biological effects. A lot of the effort in the development of green chemistry has been devoted to finding substitutes for organohalide compounds. A 2001 United Nations treaty formulated by approximately 90 nations in Stockholm, Sweden, designated a “dirty dozen” of 12 organohalide compounds of special concern as persistent organic pollutants(POP); other compounds have subsequently been added to this list.
Organosulfur and Organophosphorus Compounds
A number of organosulfur and organophosphorus compounds have been synthesized for various purposes including pesticidal applications. A common class of organosulfur compounds consists of the thiols, the simplest of which is methanethiol:
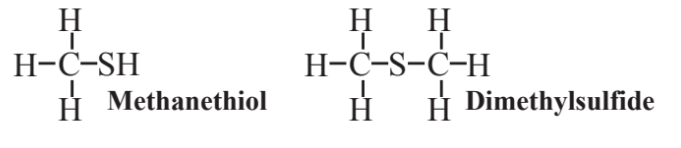
As with other thiols, which contain the -SH group, this compound is noted for its foul odor. Thiols are added to natural gas so that their odor can warn of gas leaks. Dimethylsulfide, also shown above, is a volatile compound released by ocean-dwelling microorganisms to the atmosphere in such quantities that it constitutes the largest flux of sulfur-containing vapors from Earth to the atmosphere.
Among the most prominent organophosphorus compounds are the organophosphates as shown by methyl parathion and malathion (below). These compounds are both insecticides and contain sulfur as well as phosphorus. Parathion was developed during the 1940s and was once widely used as an insecticide in place of DDT because parathion is very biodegradable, whereas DDT is not and undergoes bioaccumulation and biomagnification in ecosystems. Unfortunately, parathion has a high toxicity to humans and other animals and some human fatalities have resulted from exposure to it. Like other organophosphates, it inhibits acetylcholinesterase, an enzyme essential for nerve function (the same mode of action as its deadly cousins, the “nerve gas” military poisons, such as Sarin). Because of its toxicity, parathion is now banned from general use. Malathion is used in its place and is only about 1/100 as toxic as parathion to mammals because they — though not insects — have enzyme systems that can break it down.