6.5: Giant Molecules from Small Organic Molecules
- Page ID
- 285311
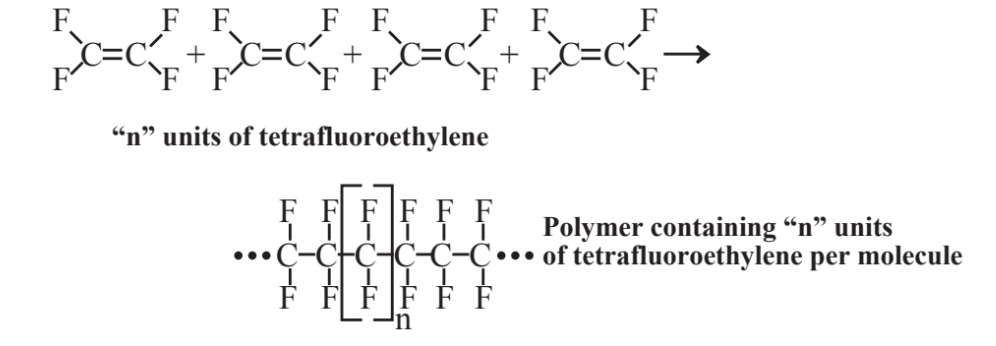
Reaction 6.2.4 showed the bonding together of molecules of ethylene to form larger molecules. This process, widely practiced in the chemical and petrochemical industries, is called polymerization and the products are polymers. Many other unsaturated molecules, usually based upon alkenes, undergo polymerization to produce synthetic polymers used as plastics, rubber, and fabrics. As an example, tetrafluoroethylene polymerizes as shown in Figure 6.6 to produce a polymer (Teflon) that is exceptionally heat- and chemical-resistant and that can be used to form coatings to which other materials will not stick (for example, frying pan surfaces).
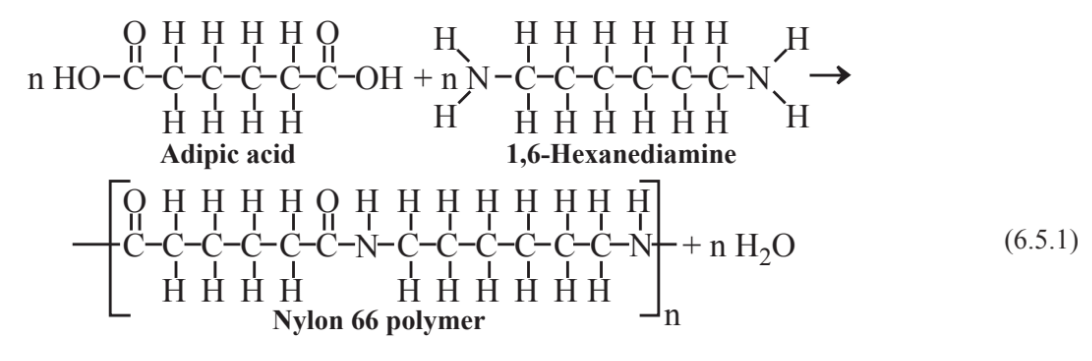
Polyethylene and polytetrafluoroethylene are both addition polymers in that they are formed by the chemical addition together of the monomers making up the large polymer molecules. Other polymers are condensation polymers that join together with the elimination of a molecule of water for each monomer unit joined. A common condensation polymer is nylon, which is formed by the bonding together of two different kinds of molecules. There are several forms of nylon, the original form of which is nylon 66 discovered by Wallace Carothers, a DuPont chemist, in 1937 and made by the polymerization of adipic acid and 1,6-hexanediamine:
There are many different kinds of synthetic polymers that are used for a variety of purposes. Some examples in addition to the ones already discussed in this chapter are given in Table 6.1.
Polymers and the industries upon which they are based are of particular concern in the practice of green chemistry for a number of reasons. The foremost of these is because of the huge quantities of materials consumed in the manufacture of polymers. In addition to the enormous quantities of ethylene and propylene previously cited in this chapter, the U.S. processes about 1.5 billion kg of acrylonitrile, 5.4 billion kg of styrene, 2.0 billion kg of butadiene, and 1.9 kg of adipic acid (for nylon 66) each year to make polymers containing these monomers. These and similarly large quantities of monomers used to make other polymers place significant demands upon petroleum resources and the energy, materials, and facilities required to make the monomers.
Table 6.1. Some Typical Polymers and the Monomers from Which they Are Formed
| Monomer | Monomer Formula | Polymer | Applications |
| Propylene (polypropylene) | 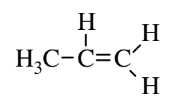 |
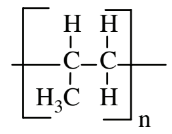 |
Applications requiring harder plastic, luggage, bottles, outdoor carpet |
| Vinyl chloride (polyvinyl chloride) | 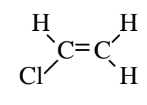 |
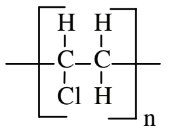 |
Thin plastic wrap, hose, flooring, PVC pipe |
| Styrene (polystyrene) | 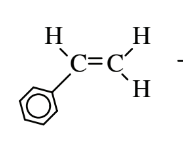 |
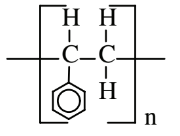 |
Plastic furniture, plastic cups and dishes, blown to produce styrofoam plastic products |
| Acrylonitrile (polyacrylonitrile) | 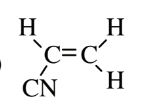 |
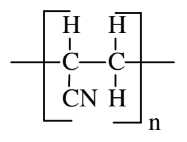 |
Synthetic fabrics (Orlon, Acrilan, Creslan), acrylic paints |
| Isoprene (polyisoprene) | 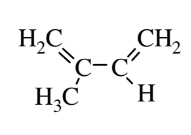 |
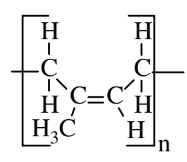 |
Natural rubber |
There is a significant potential for the production of pollutants and wastes from monomer processing and polymer manufacture. Some of the materials contained in documented hazardous waste sites are byproducts of polymer manufacture. Monomers are generally volatile organic compounds with a tendency to evaporate into the atmosphere, and this characteristic combined with the presence of reactive C=C bonds tends to make monomer emissions active in the formation of photochemical smog (see Chapter 10). Polymers, including plastics and rubber, pose problems for waste disposal, as well as opportunities and challenges for recycling. On the positive side, improved polymers can provide long-lasting materials that reduce material use and have special applications, such as liners in waste disposal sites that prevent waste leachate migration and liners in lagoons and ditches that prevent water loss. Strong, lightweight polymers are key components of the blades and other structural components of huge wind generators that are making an increased contribution to renewable energy supplies around the world (see Chapter 16).
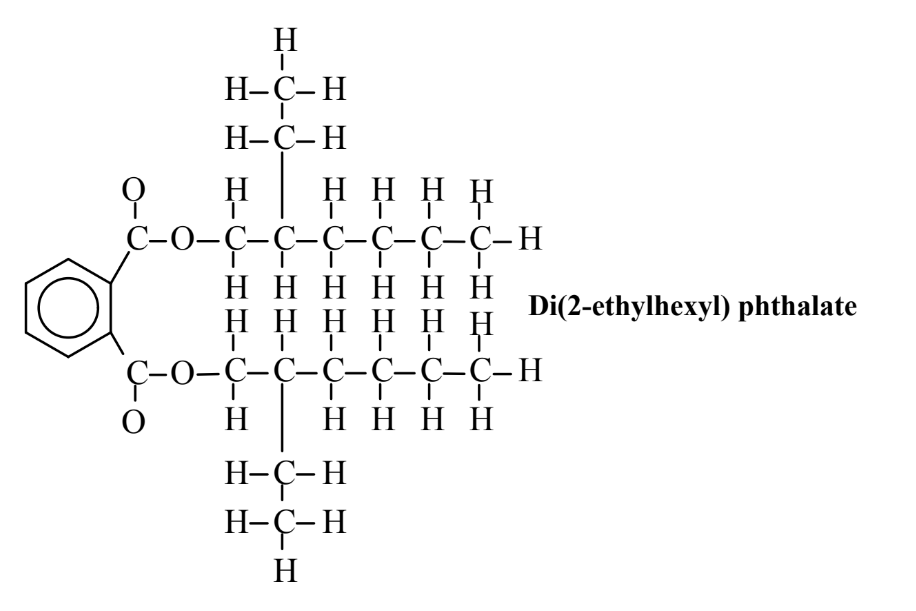
Some of the environmental and toxicological problems with polymers have arisen from the use of additives to improve polymer performance and durability. The most notable of these are plasticizers, normally blended with plastics to improve flexibility, such as to give polyvinylchloride the flexible characteristics of leather. The plasticizers are not chemically bound as part of the polymer and they leak from the polymer over a period of time, which can result in human exposure and environmental contamination. The most widely used plasticizers are phthalates, esters of phthalic acid as shown by the example of di(2-ethylhexyl) phthalate below. Though not particularly toxic, these compounds are environmentally persistent, resistant to treatment processes, and prone to undergo bioaccumulation. They are found throughout the environment and have been implicated by some toxicologists as possible estrogenic agents that mimic the action of female sex hormone and cause premature sexual development in young female children.