4.3: Sodium Chloride and Ionic Bonds
- Page ID
- 284497
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Many atoms and groups of atoms in chemical compounds are ions that have an electrical charge because of their unequal numbers of protons and electrons. Cations are positively charged ions and anions are negatively charged ions. Compounds consisting of ions are ionic compounds and the bonds holding them together are ionic bonds. Ionic bonds depend upon the mutual attraction between positive cations and negative anions for their bond strength (oppositely charged bodies attract each other, whereas negatively charged bodies repel each other).
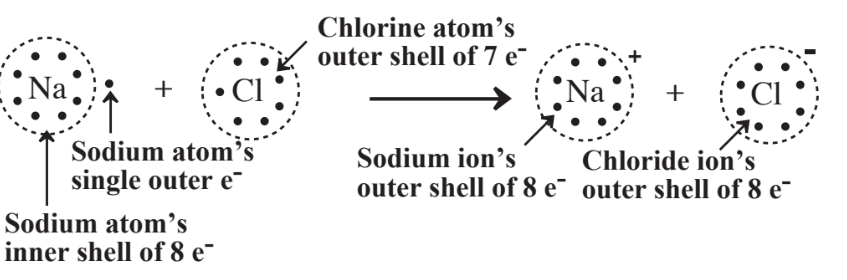
The formation of ions based upon the octet rule is readily seen for the well-known ionic compound, sodium chloride, NaCl, as illustrated in Figure 4.3. By losing an electron to become the Na+ cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. Chlorine attains a stable octet of 8 outer-shell electrons by gaining 1 electron per atom to produce Cl- ion.
Sodium chloride is a very stable compound because of the mutual attraction of oppositely charged ions. But the ions have to be arranged in an optimum manner for this attraction to be effective. Since oppositely charged ions attract each other, but ions with the same charge are mutually repulsive, the ions in an ionic compound such as sodium chloride have to be packed to maximize attraction and minimize repulsion. The arrangement that does this for NaCl is shown by a ball and stick model in Figure 4.4.
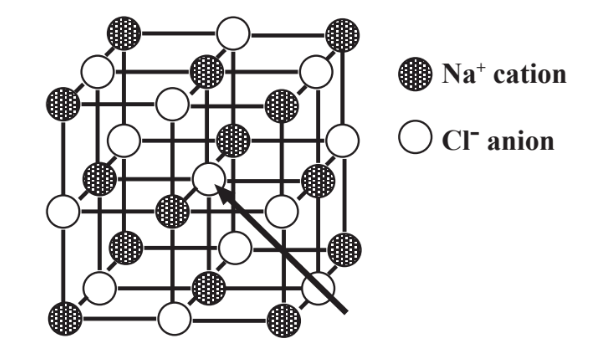
Although it may be a little hard to imagine for a model represented on paper, the six nearest neighbors of each negatively charged Cl- anion are Na+ cations. And the six nearest neighbors of each positively charged Na+ cation are negatively charged Cl- anions
In reality, ions are more accurately represented in an ionic structure as spheres that touch. The Na+ cation is significantly smaller than the Cl- anion, so a more accurate representation of NaCl than that shown in Figure 4.4 would show rather large Cl- spheres between which are nestled barely visible Na+spheres. But the imperfect ball and stick model shown in Figure 4.4 shows several important points regarding ionic NaCl. It illustrates the relative positions of the ions. These positions, combined with ionic charge and size, determine the crystal structure of the solid crystal of which the ionic compound is composed. Furthermore, examination of the figure shows that no single Cl- anion belongs to a specific Na+ cation, and no single Na+ cation belongs to a specific Cl- anion. So, although the chemical formula NaCl accurately represents the relative numbers of Na and Cl atoms in sodium chloride it does not imply that there are discrete molecules consisting of 1 Na and 1 Cl. For this reason it is not correct to refer to a molecule of sodium chloride because distinct molecules of ionic compounds do not exist as such. Instead, reference is made to formula units of ionic compounds, where a formula unit of NaCl consists of 1 Na+ ion and 1 Cl- ion, the smallest quantity of a substance that can exist and still be sodium chloride.
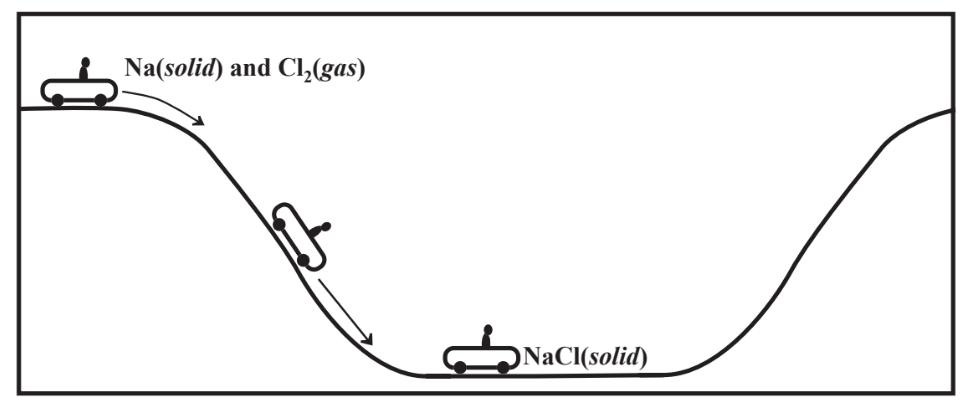
The stabilities of chemical compounds are all about energy. In general, the more energy released when a compound forms, the more stable the compound is. Sodium chloride could be formed by reacting elemental solid sodium with elemental Cl2 gas,
\[\ce{2Na(solid) + Cl2(gas) \rightarrow 2NaCl (solid)}\]
to produce solid sodium chloride. This reaction releases a large amount of energy and elemental sodium burns explosively in chlorine gas. The reaction can be viewed in terms of the following steps.
- The atoms in solid Na are taken apart, which requires energy.
- Each molecule of Cl2 is taken apart, which requires energy.
- An electron is taken from each Na atom to produce Na+ ion, which requires energy.
- An electron is added to each Cl atom to produce a Cl- ion, which releases energy.
- All the Na+ cations and 1 Cl- anion are assembled in a 1/1 ratio in a crystal lattice to produce NaCl, which releases a very large quantity of energy.
The very large amount of energy involved in Step 5 is called the lattice energy and is primarily responsible for the high stability of ionic compounds. A general picture of the energy involved is shown in Figure 4.5.
The differences in ionic size noted above are represented in Figure 4.6 for several monatomic (1-atom) ions from elements close to each other in the periodic table. The figure shows that negative monatomic ions are generally larger than positive monatomic ions formed from elements that are nearby in the periodic table. Thus, the negative F- ion is larger than the positive Na+ion, although both ions have the same number of electrons (10) and the atomic number of Na is higher than that of F. It is
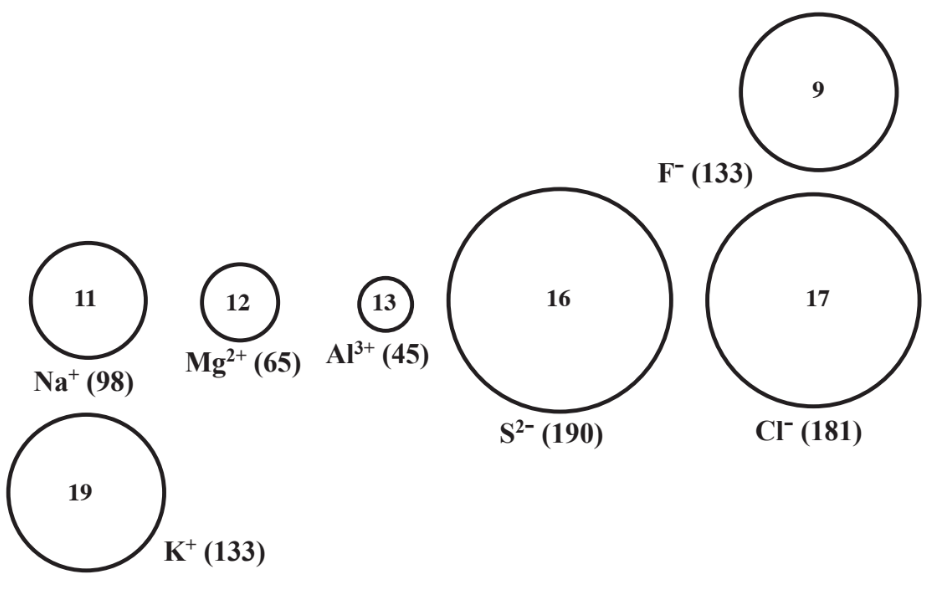
seen that for ions in the same group of elements that have the same charge, the ion from the element with higher atomic number is larger. Figure 4.6 shows the Cl-ion larger than the F- ion and the K+ ion is larger than the Na+ ion. As electrons are removed from elements in the same period of the periodic table to produce progressively more highly charged cations, ion size shrinks notably as shown by the order of ion sizes Na+> Mg2+> Al3+, each of which has 10 e-. This occurs because as the charge of the nucleus becomes larger relative to the charge of the negative electron cloud around it, the cloud is drawn closer to the nucleus, shrinking ion size. As electrons are added to atoms to produce more highly charged anions, the anion size increases because more electrons occupy more space. So S2- ion is larger than Cl- ion.
In order to further understand ion formation, several more examples can be considered. Calcium and chlorine react,
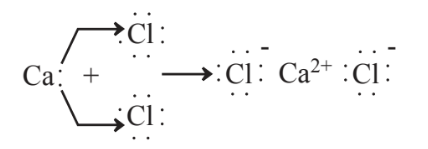
to form calcium chloride, CaCl2. This compound is a byproduct of some industrial processes, from which its disposal can be a problem. It is commonly used as road salt to melt ice and snow on streets and highways. Although calcium chloride is effective in this respect, it is corrosive to automobiles and calcium chloride is a pollutant salt that can contribute to excess salt levels in bodies of water. A “greener,” though more costly substitute is calcium acetate, Ca(C2H3O2)2. This compound is composed of Ca2+ ions and acetate (C2H3O22-) anions. Its advantage is that bacteria on soil and in water readily cause biodegradation of the acetate anion as shown by the reaction,
\[\ce{Ca(C2H3O2)2 + 4O2} \: \: \: \underrightarrow{Bacteria} \: \: \: \ce{CaCO3 + 3CO2 + 3H2O}\]
from which the calcium ends up as calcium carbonate, a common component of rock and soil.
Another example of the formation of an ionic compound is the following reaction of aluminum metal with elemental oxygen,
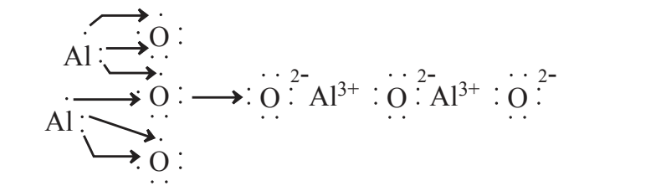
This reaction produces aluminum oxide for which the chemical formula is Al2O3. This compound is the source of aluminum in bauxite, the ore from which aluminum is produced and is an important industrial chemical. Called alumina, aluminum oxide itself has many applications including its use for abrasives and sandpaper, as a raw material for ceramics, and as an ingredient of antacids and antiperspirants.
Exercise: Show the ionic products of the reaction of the metal and nonmetal indicated

Answers: (a) Na+, (b) Cl-, (c) NaCl, (d) K+, (e) O2-, (f) K2O, (g) Ca2+, (h) Cl-, (i) CaCl2
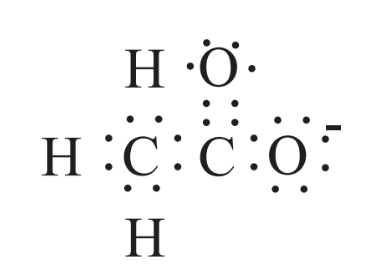
In addition to ions formed from single atoms losing or gaining electrons, many ions consist of groups of atoms covalently bound together, but having a net electrical charge because of an excessor a deficiency of electrons. An example of such an ion is the acetate ion shown above in the formula of calcium acetate, Ca(C2H3O2)2. The structural formula of the acetate anion, C2H3O2-, is shown below in which the two carbon atoms are joined with a single covalent bond consisting of two shared electrons, each of the three H atoms are joined to one of the carbon atoms by a single covalent bond and the other carbon atom is joined to one oxygen with a single covalent bond and to the other by a double covalent bond consisting of 4 shared electrons. The net charge on the ion is -1.
Ionic Liquids and Green Chemistry
Most common ionic compounds such as sodium chloride are hard solids because the ions of which they are composed are relatively small and packed tightly together in the crystalline lattice. These ionic compounds must be heated to very high temperatures before they melt, 801 ̊C for NaCl, for example. In recent years, ionic compounds have been developed that are liquids under ordinary conditions. The ions in these ionic liquids are composed of large organic molecules composed of skeletons of numerous carbon atoms bonded to other atoms and having a net charge. The charges on the ions in such compounds is much less concentrated than in simple inorganic compounds like NaCl, the large ions move readily relative to each other in the ionic crystal, and the compound is liquid at low temperatures. A common example of an ionic liquid compound is decylmethylimidazolium hexafluorophosphate, which is a liquid at temperatures above 40 ̊C, the temperature of a very hot summer’s day.
There has been a lot of interest in the application of ionic liquids to green chemistry. This is because many chemical reactions including those for preparing polymers used in synthetic fabrics or pharmaceutical compounds are carried out in liquid solvents, which tend to evaporate and contaminate air and to pose disposal problems. Furthermore, although the solvent properties in chemical synthesis often play a strong role in determining the course of the synthesis, the number of available solvents is very limited. In the case of ionic liquids, however, there is a vast variety of both cations and liquids which, combined together, could give as many as a trillion (!) different ionic liquids. This versatility enables fine-tuning the properties of the ionic liquids for specialized uses in synthesis and other applications. The ionic liquids are rather easy to recycle, adding to their green character. In addition to their applications as solvents for chemical synthesis media, ionic liquids may be useful for isolating pollutants. For example, an appropriate ionic liquid may be shaken with water contaminated with toxic heavy metals, such as lead or cadmium, which bind with the ionic liquid. Since such a liquid is normally not soluble in water, it can be physically separated from the water, carrying the heavy metals with it and leaving purified water.


