4.2: Electrons Involved in Chemical Bonds and Octets of Electrons
- Page ID
- 284485
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The electrons in the outermost shell of atoms are those that become involved in chemical bonds. These are called valence electrons. Refer back to the Lewis symbols of the elements shown in Figure 3.9. Note that the three elements on the right of the table are noble gases that are chemically content with their filled outer electron shells containing 2 electrons in the case of helium and 8 each for neon and argon. As a basis for the understanding of chemical bonds consider that the other elements tend to attain the filled electron shells of their nearest-neighbor noble gases by sharing, losing, or gaining electrons. Of these elements, the only one that we will consider in detail that attains a helium-like electron configuration is hydrogen, H, each atom of which almost always has access to 2 electrons shared in covalent bonds. The other elements that we will consider, carbon and higher, attain 8 electrons in their outer shells by chemical bonding. This is the basis of the octet rule, the tendency of atoms to attain stable outer shells of 8 electrons by forming chemical bonds. The octet rule is immensely useful in explaining and predicting chemical bonds and the formulas and structures of chemical compounds and will be emphasized in this chapter.
Some examples of the kinds of bonding arrangements discussed above have already been illustrated in Chapter 3. Figure 3.1 illustrates that, even in the elemental form, H2, hydrogen atoms have 2 valence electrons in the diatomic molecule. Examples of elements that have 8 valence electrons as the result of chemical bonding were also shown. Figure 3.6 illustrates the two N atoms in the N2 molecule sharing 6 electrons in a covalent bond so that each of the atoms may have an octet. Figure 3.8 shows that 2 Cl atoms, each with 7 valence electrons, share 2 electrons in the covalent bond of the Cl2 molecule to attain octets. The same figure shows that Na loses its single valence electron in forming ionic NaCl to produce the Na+ ion, which has an octet of electrons in its outer shell. In forming the same ionic compound, Cl gains an electron to become the Cl- anion, which also has a stable octet of outer-shell electrons.
In the remainder of this chapter, the octet rule will be used in explaining the formation of chemical compounds consisting of two or more different elements bonded together. It was already used to show the bonding in ionic sodium chloride in Figure 3.8. One of the best compounds for showing the octet rule in covalent compounds is methane, CH4, shown in Figure 4.1. The molecule of CH4 is produced when an atom of carbon with 4 outer electrons (see Figure 3.9) attains an octet of 8 electrons by sharing with H atoms.
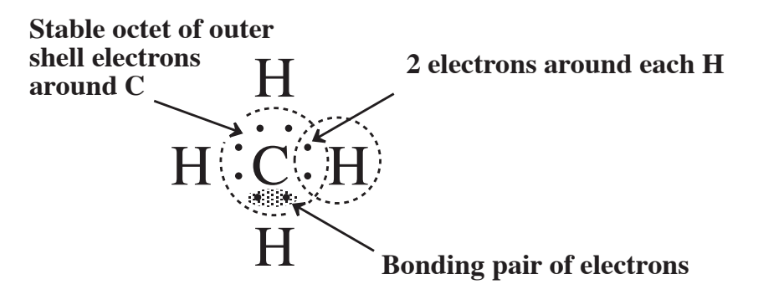
Each H atom has 1 electron to donate to the sharing arrangement, so by each of 4 H atoms contributing an electron the carbon atom can gain an octet. Each of the H atoms has access to 2 electrons in the single covalent bond that connects it to the C atom. Examination of Figure 4.1 implies that the 4 H atoms and the C atom all lie in the same plane in a flat structure. But that is not the case because of the tendency for the 4 electron pairs composing the 4 covalent bonds to be oriented as far as possible from each other around the sphere of the carbon atom. The resulting structure is a little hard to illustrate on paper, but one way to approximate it is with a ball and stick model that represents the atoms as balls and the chemical bonds as sticks connecting the atoms. Figure 4.2 is an illustration of the ball and stick model of CH4.


