1.3: Formation and evolution of the Earth
- Page ID
- 98748
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The solar system is believed to have formed about 5 billion years ago as a result of aggregation of cosmic dust and interstellar atoms in a region of space in which the density of such material happened to be greater than average. Over 99.8% of this mass, which consisted mostly of hydrogen, collapsed into a proto-sun; the gravitational energy released in this process raised the temperature sufficiently to initiate the hydrogen fusion reactions discussed above.
The planets
The remaining material probably formed a disk that rotated around the sun. As the temperature dropped to around 2000K, some of the most stable combinations of the elements began to condense out. These substances might have been calcium aluminum silicates, followed by the more volatile iron-nickel system, and then magnesium silicates. The further aggregation of these materials, together with the other constituents of the cooling disk, is now believed to be the origin of the planets. Density estimates indicate that the planets closest to the sun are predominantly rocky in nature, and probably condensed first. The outer planets (Uranus, Neptune and Pluto) appear to consist largely of water ice, methane, and ammonia, with a smaller rocky core.
Formation of the Earth
The Earth formed by accretion of solid and particulate material that remained after the much more massive amounts of hydrogen and helium present in the original protoplanets had been dispersed out of the solar system. Gradually, the heat produced by decay of radioactive elements brought about partial melting of the silicate rocks; these lower density molten materials migrated upward, leaving the more dense, iron-containing minerals below. This process, which took about 2 million years, was the first of the three stages into which the chemical evolution of the earth is usually divided:
- Primary differentiation of the elements between the core and mantle.
- Secondary differentiation of the elements, reflecting relative ionic sizes, bonding properties, and solubilities (influencing phase behavior such as fractional crystallization, etc.)
- Tertiary differentiation, still operative, involving the interaction of the crust with the hydrosphere and atmosphere.
The above listing should not be taken too literally; all three kinds of processes have probably proceeded simultaneously, and over a number of cycles. Since the earth is losing approximately four times as much heat as is generated by radioactive decay, the principal driving force of primary and secondary differentiation has gradually slowed down. Partial melting of the upper mantle brought about further fractionation as silicon-containing materials of low density migrated outward to form a crust. In its early stages the stronger granitic rocks had not yet appeared, and the crust was mechanically weak. Upwelling flows of lava would break the surface, and the weight of the solidified lava would cause the crust to subside. In some places, magma would solidify underground, forming low-density rock (batholiths) that would eventually rise by buoyancy and push up overlying crust. These mountain-building periods probably occurred in 6-8 major episodes, each lasting about 800 million years.
Rain
At the same time, outgassing of solids released large amounts of HCl, CO, CO2, H2S, CH4 , SO2 , and SO3 into the primitive atmosphere. Large amounts of water were present in the primeval rocks in the form of hydrates, which were broken down as the result of the heating. Eventually when the outer crust cooled enough to permit condensation of the water vapor as rain, a new stage of chemical evolution began. The rain was initially highly acidic, equivalent to about 1M HCl; this reacted readily with the basic rocks having high contents of K, Na, Mg, and Ca, leaching them away and forming what would eventually evolve into the oceans. The partial dissolution of the rocks also resulted in large amounts of sediments, which played their own role in the transformation of the earth’s surface.
The continents
Within the crust, the lighter materials, being in isostatic equilibrium with the upper mantle, floated higher, and gradually became the nuclei of continents, which grew by accumulating similar material around their boundaries. This picture of continental development is supported by isotopic ratio studies which indicate that the nucleus of the North American continent, the Canadian Shield, is over 2.5 billion years old, while the peripheral parts are less than 0.6 billion years of age.
Primary differentiation of the elements
The more traditional geochemical view of primary differentiation begins with the assumption that the core of the earth is in a chemically reduced state, while the metallic elements constituting the mantle are almost entirely oxidized to their lower free energy cationic forms. Oxygen and sulfur acted as the major electron acceptors in this process, but the abundance of these elements was insufficient to oxidize much of the nickel or iron.
Iron as a reductant
Iron itself is believed to have played a crucial role in the primary differentiation of other metals and of oxidized metallic elements that iron is able to reduce. As the dense molten iron migrated in toward the core, it dissolved (formed a liquid alloy with) any other metals with which it came in contact, and it reduced (donated electrons to) those metallic cations that are less “active” metals than iron under these conditions. The resulting metal would then mix with more of the migrating liquid iron, and be carried along with it into the core.
Redox power of the elements
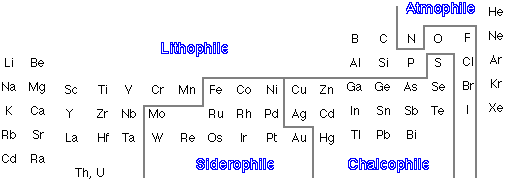
Accordingly, elements whose reduction potentials are more positive than iron (i.e., are lower-free energy electron sinks) are called siderophiles; these elements have a low abundance in the crust and upper mantle. The other two important classes of solid-forming elements are lithophile and chalcophile (see below.) These generally have more negative reduction potentials than iron, and are distinguished mainly by their relative affinity for oxygen or sulfur. The chalcophiles, of which Cu, Cd, and Sb are examples, tend to form larger, more polarizable ions which can associate with the sulfide ion. The lithophiles comprise those elements such as K, Al, Mn, and Si, which have smaller ions and which combine preferentially with oxygen. This broad classification is reflected in the dominant forms in which many of these elements occur in nature.
Secondary differentiation of the elements
The differential distribution of the elements within one of the main regions of the earth has been studied in detail only in that portion that is accessible, namely the upper crust. It is clear that fractional crystallization from the cooling magma has played an important role. The relative temperatures at which minerals crystallize is determined in large part by their lattice energies, which are in turn related to ionic sizes and charges. Minerals with small, highly charged ions will have higher melting points and should crystallize first. Thus the sodium-containing feldspar albite (NaAlSi2O8) is found nearer the surface than is its calcium analog anorthite (CaAlSi2O8). The less abundant elements often do not form minerals of their own, but may replace the ion of a more abundant mineral in its crystal lattice. This is known as isomorphous replacement, and it naturally depends on the relative ionic radii. Some ion pairs that undergo isomorphous replacement in minerals are K+ and Ba2+, Si4+ and Ge4+.
Phase behavior
The Phase Rule can be invoked to explain in a very rough way the differentiation of the elements into distinct solid phases.
P = C + 2 – F
Taking the degrees of freedom as 2 (fixed temperature and pressure), the six major elemental components (O, Si, Al, Fe, Mg and Na) can form up to six phases. Actually, more than 99% of igneous rocks comprise seven principal mineral phases. These are: the silica minerals, feldspars, feldspathoids, olivine, pyroxenes, amphiboles and micas.
The differential deposition of minerals is also influenced by the temperature-composition phase relations as exemplified by the ordinary two-component phase diagram. If the mineral that is rich in one component and which first crystallizes out is also more dense, then the richer ore will occur near the bottom of the deposit, while a more mixed ore (approaching the eutectic) will remain near the top.
Geochemical classification of the elements
Whether an element is concentrated in the crust or elsewhere depends on its chemical behavior and on the physical properties of its stable compounds. Geochemists have found it convenient to establish the following general classifications:
- lithophiles ("rock-loving") elements are those such as Fe, Al, and Si which tend to occur as oxides (and to a lesser extent as chlorides and carbonates.) Elements in this, the largest of all the groups, are concentrated in the crust.
- chalcophiles also occur in the crust, but mainly in combination with sulfur and the other chalcogen elements of Groups 15-16.
- siderophiles refer to the elements such as Ni which have concentrated in the core along with Fe.
- atmosphiles consist of N, H and their volatile compounds
and the noble gases which concentrate in the atmosphere.
 |
||
References
"TheTalk" site contains numerous links to discussions of geochronology and other evidence for the age of the earth, and on the pseudoscientific concept of a "young Earth".
Unravelling geological time (Columbia University)
Formation of the Earth's core - description of theories based on high pressure studies
Page last modified: 21.01.2008
© 1998 by Stephen Lower
For information about this Web site or to contact the author,
please see theChem1 Virtual Textbook home page.

This work is licensed under a Creative Commons Attribution-NonCommercial 2.5 License.


