1.2: Origin of the Elements
- Page ID
- 98749
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Earth is composed of 90 chemical elements, of which 81 have at least one stable isotope. Most of these elements have also been detected in stars. Where did these elements come from? The accepted scenario is that the first major element to condense out of the primordial soup was helium, which still comprises about one-quarter of the mass of the known universe.

Primordial Chemistry
According to the “big bang” theory for which there is now overwhelming evidence, the universe as we know it (that is, all space, time, and matter) had its origin in a point source or singularity that began an explosive expansion about 12-15 billion years ago, and which is still continuing.
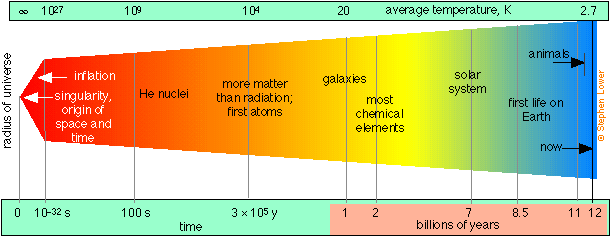
Following a brief period of extremely rapid expansion called inflation, protons and neutrons condensed out of the initial quantum soup after about 10–32 s. Helium and hydrogen became stable during the first few minutes, along with some of the very lightest nuclides up to 7Li, which were formed through various fusion and neutron-absorption processes. Formation of most heavier elements was delayed for about 106 years until nucleosynthesis commenced in the first stars. Hydrogen still accounts for about 93% of the atoms in the universe.
Stellar Nucleosynthesis
All elements beyond hydrogen were formed in regions where the concentration of matter was large, and the temperature was high; in other words, in stars. The formation of a star begins when the gravitational forces due to a large local concentration of hydrogen bring about a contraction and compression to densities of around 105 g cm–3. This is a highly exothermic process in which the gravitational potential energy is released as heat, about 1200 kJ per gram, raising the temperature to about 107 K. Under these conditions, hydrogen nuclei possess sufficient kinetic energy to overcome their electrostatic repulsion and undergo nuclear fusion:
\[\ce{4 ^{1}_1H -> ^{4}_2He + 2 \beta^{+} + 2\gamma + 2 n}\]
Hydrogen burning
There will be a net mass loss in above process, which will therefore be highly exothermic and is known as “hydrogen burning”. As hydrogen burning proceeds, the helium collects in the core of the star, raising the density to 108 g cm–3 and the temperature to 108 K. This temperature is high enough to initiate helium burning, which proceeds in several steps:
\[\ce{2 ^{4}_2He -> 4Be8 + g}\]
The first product, \(\ce{^{8}_4Be}\) has a half life of only 10–16 sec, but a sufficient amount accumulates to drive the following two reactions:
\[ \ce{^{8}_4Be + ^{4}_2He -> ^{12}_6C + \gamma}\]
\[ \ce{ ^{12}_6C + ^{1}_1H -> ^{13}_1N + ^{13}_1C + \beta^{+} + \gamma}\]
The size of a star depends on the balance between the kinetic energy of its matter and the gravitational attraction of its mass. As the helium burning runs its course, the temperature drops and the star begins to contract. The course of further nucleosynthesis and the subsequent fate of the star itself depends on the star’s mass.
Small stars
If the mass of the star is no greater than 1.4 times the mass of our sun, the star collapses to a white dwarf, and eventually cools to a dark, dense dead star.
Big stars
In larger stars, the gravitational forces are sufficiently strong to overcome the normal repulsion between atoms, and so gravitational collapse continues. The gravitational energy released in this process produces temperatures of 6  108 K, which are sufficient to initiate a complex series of nuclear reactions known as the carbon-nitrogen cycle. The net reaction of this cycle is the further fusion of hydrogen to helium, in which C12 acts as a catalyst, and various nuclides of nitrogen and oxygen are intermediates. The temperature is sufficiently high, however, to initiate fusion reactions of some of these intermediates:
108 K, which are sufficient to initiate a complex series of nuclear reactions known as the carbon-nitrogen cycle. The net reaction of this cycle is the further fusion of hydrogen to helium, in which C12 acts as a catalyst, and various nuclides of nitrogen and oxygen are intermediates. The temperature is sufficiently high, however, to initiate fusion reactions of some of these intermediates:
6C12 + 6C12 \( \rightarrow \) 10Ne20 + 2He4
2 8O16 \( \rightarrow \) 14Si28 + 2He4
2 8O16 \( \rightarrow \) 16S31 + 0n1
Supernovas
The intense gamma radiation that is produced in some of these reactions breaks some of the product nuclei into smaller fragments, which can then fuse into a variety of heavier species, up to the limit of 26Fe56, beyond which fusion is no longer exothermic. The greater relative abundance of elements such as 6C12, 8O16, and 10Ne20 which differ by a 2He4 nucleus, reflects the participation of the latter species in these processes. These exothermic reactions eventually produce temperatures of 8  109 K, while contraction continues until the central core is essentially a ball of neutrons having a radius of about 10 km and a density of 1014 g cm–3. At the same time the outer shell of the star is blasted away in an explosion known as a supernova.
109 K, while contraction continues until the central core is essentially a ball of neutrons having a radius of about 10 km and a density of 1014 g cm–3. At the same time the outer shell of the star is blasted away in an explosion known as a supernova.
Note
Only six supernovas have been observed in our galaxy. The supernova of 1987 was the most recent; the one before this occurred in 1604, prior to the invention of the telescope. Tycho Brahe's observation of a supernova in 1572 was crucial in overturning the Aristotelian tradition of the immutability of the "fixed stars", or "firmament". The remains of these supernovas have been detected and studied by X-ray observations.
Thus all of the elements in our solar system that are heavier than iron are the recycled remnants of former stars.
Elements heavier than iron
Since 26Fe56 has the highest binding energy per nucleon of any nuclide, there are no exothermic processes which can lead to the formation of heavier elements. Fusion into heavier species is also precluded by the electrostatic repulsion of the highly charged nuclei. However, the process of neutron capture can still take place (this is the same process that is used to make synthetic elements). The neutrons are by-products of a large variety of stellar processes, and are present in a wide range of energies. Two general types of neutron capture processes are recognized. In an “s” (slow) process, only a single neutron is absorbed and the product usually decomposes by b-decay into a more proton-rich species:
26Fe56 + 0n1 \( \rightarrow \) 26Fe56 \( \rightarrow \) 26Fe59 \( \rightarrow \) 27Co59 + –1e0
This process occurs at rates of about 105 yr–1 , and accounts for the lighter isotopes of many elements. The other process (the “r”, or rapid process) occurs in regions of high neutron density and involves multiple captures at rates of 0.1-10 sec–1:
26Fe56 + 13 0n1 \( \rightarrow \) 26Fe56 \( \rightarrow \) 27Co59 + –1e0
This mechanism favors the heavier, neutron-rich isotopes and the heaviest elements.
Other elements
A few nuclei are not accounted for by any of the processes mentioned. These are all low-abundance species, and they probably result from processes having low rates. Examples are Sn112 and Sn114 which may be produced through proton-capture, and H2, Li6, Li7, Be, B10 and B11, which may come from spallation processes resulting from collisions of cosmic ray particles with heavier elements.
Contributors
Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook


