5.7: Community ecotoxicology
- Page ID
- 294568
5.7. Community ecotoxicology
5.7.1. Community Ecotoxicology: theory and concepts
Authors: Michiel Kraak and Ivo Roessink
Reviewers: Kees van Gestel, Nico van den Brink, Ralf B. Schäfer
Learning objectives:
You should be able to
- motivate the importance of studying ecotoxicology at the community level.
- define community ecotoxicology and to name specific phenomena at the community and ecosystem level.
- explain the indirect effects observed in community ecotoxicology.
- explain how communities can be studied and how data from experiments at the community level can be analyzed.
- interpret community ecotoxicity data and to explain related challenges.
Keywords: Community ecotoxicology, species interactions, indirect effects, mesocosm, ecosystem processes.
Introduction
The motivation to study ecotoxicological effects at the community level is that generally the targets of environmental protection are populations, communities and ecosystems. Consequently, when scaling up research from the molecular level, via cells, organs and individual organisms towards the population, community or even ecosystem level the ecological and societal relevance of the obtained data strongly increase (Figure 1). Yet, the difficulty of obtaining data increases, due to the increasing complexity, lower reproducibility and the increasing time needed to complete the research, which typically involves higher costs. Moreover, when effects are observed in the field it may be difficult to link these to specific chemicals and to identify the drivers of the observed effects. Not surprisingly, ecotoxicological effects at the community and ecosystem level are understudied.

Community Ecotoxicology: definition and indirect effects
Community ecology is defined as the study of the organization and functioning of communities, which are assemblages of interacting populations of species living within a particular area or habitat. Building on this definition, community ecotoxicology is defined as the study of the effects of toxicants on patterns of species abundance, diversity, community composition and species interactions. These species interactions are unique to the community and ecosystem level and may cause direct effects of toxicants on specific species to exert indirect effects on other species. It has been estimated that the majority of effects at these levels of biological organization are indirect rather than direct. These indirect effects are exerted via:
- predator-prey relationships
- consumer-producer relationships
- competition between species
- parasite-host relationships
- symbiosis
- biotic environment
As an example, Roessink et al. (2006) studied the impact of the fungicide triphenyltin acetate (TPT) on benthic communities in outdoor mesocosms. For several species a dose-related decrease in abundance directly after application was observed, followed by a gradual recovery coinciding with decreasing exposure concentrations, all implying direct effects of the fungicide. For some species, however, opposite results were obtained and abundance increased shortly after application, followed by a gradual decline; see the example of the Culicidae in Figure 2. In this case, these typical indirect effects were explained by a higher sensitivity of the predators and competitors of the Culicidae. Due to diminished predation and competition and higher food availability abundances of the Culicidae temporarily increased after toxicant exposure. With the decreasing exposure concentrations over time, the populations of the predators and competitors recovered, leading to a subsequent decline in numbers of the Culicidae.
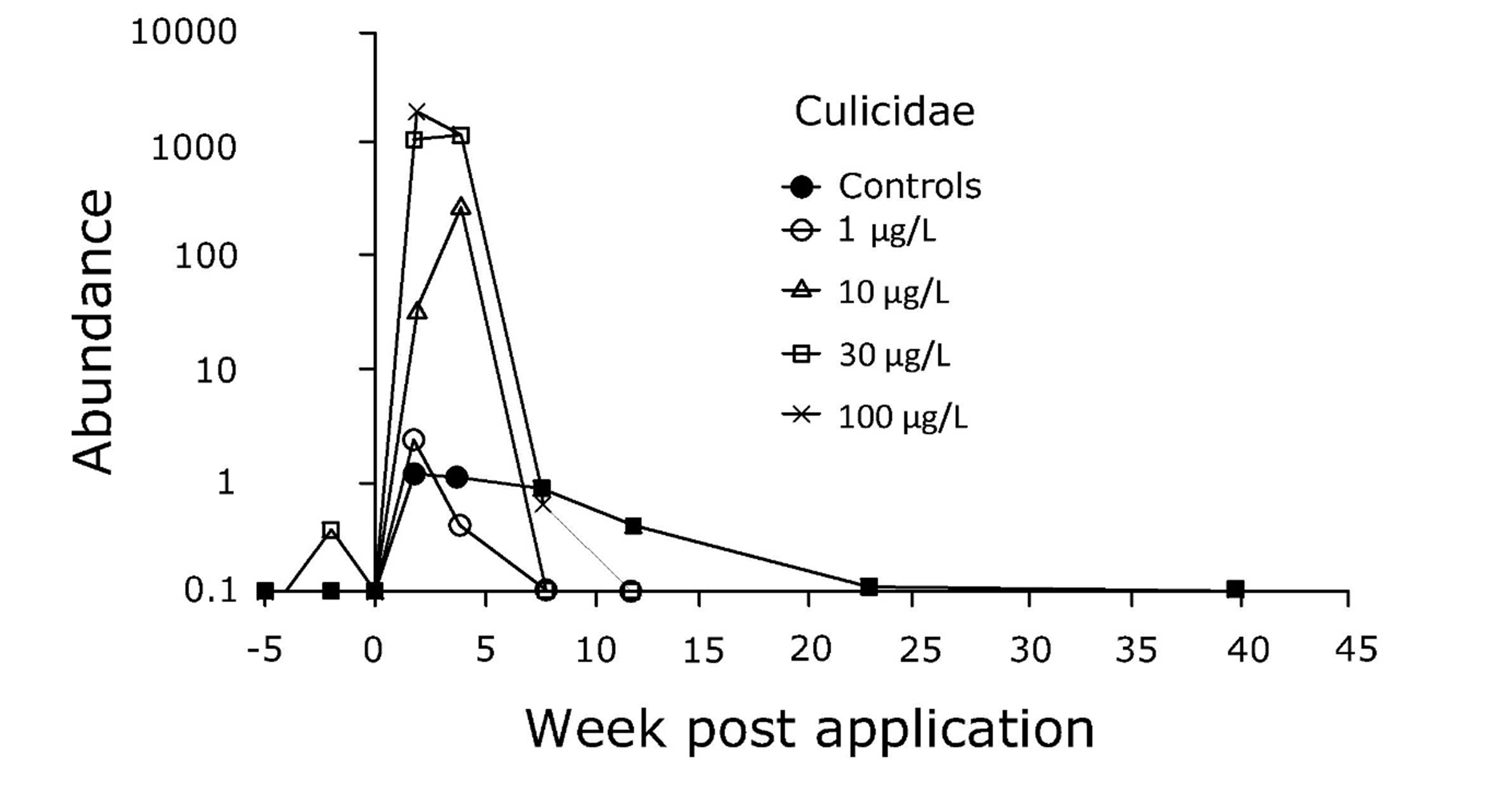
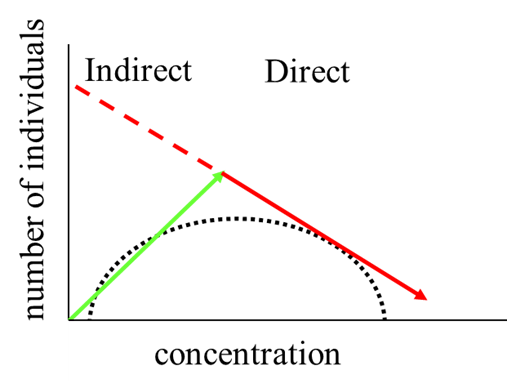
The indirect effects described above are thus due to species-specific sensitivities to the compound of interest, which influence the interactions between species. Yet, at higher exposure concentrations also the less sensitive species will start to be affected by the chemical. This may lead to an "arch-shaped" relationship between the number of individuals of a certain species and the concentration of a toxicant. In a mesocosm study with the insecticide lambda-cyhalothin this was observed for Daphnia, which are prey for the more sensitive phantom midge Chaoborus (Roessink et al., 2005; Figure 3). At low exposure concentrations the indirect effects, such as release from predation by Chaoborus, led to an increase in abundance of the less sensitive Daphnia. At intermediate exposure concentrations there was a balance between the positive indirect effects and the adverse direct effects of the toxicant. At higher exposure concentrations the adverse direct effects overruled the positive indirect effects resulting in a decline in abundance of the Daphnia. These combined dose dependent direct and indirect effects are inherent to community-level experiments, but are compound and species-interaction specific.
Investigating communities and analysing and interpreting community ecotoxicity data
To study community ecotoxicology, experiments have to be scaled up and are therefore often performed in mesocosms, artificial ponds, ditches and streams, or even in the field, sometimes accompanied by the use of in- and exclosures. To assess the effects of toxicants on communities in such large systems requires meticulous sampling schemes, which often make use of artificial substrates and e.g. emergence traps for aquatic invertebrates with terrestrial adult life stages (see section on Community ecotoxicology in practice).
Alternatively to scaling up the experiments in community ecotoxicology, the size of the communities may be scaled down. Algae and bacteria grown on coin sized artificial substrates in the field or in experimental settings provide the unique advantage that the experimental unit is actually an entire community.
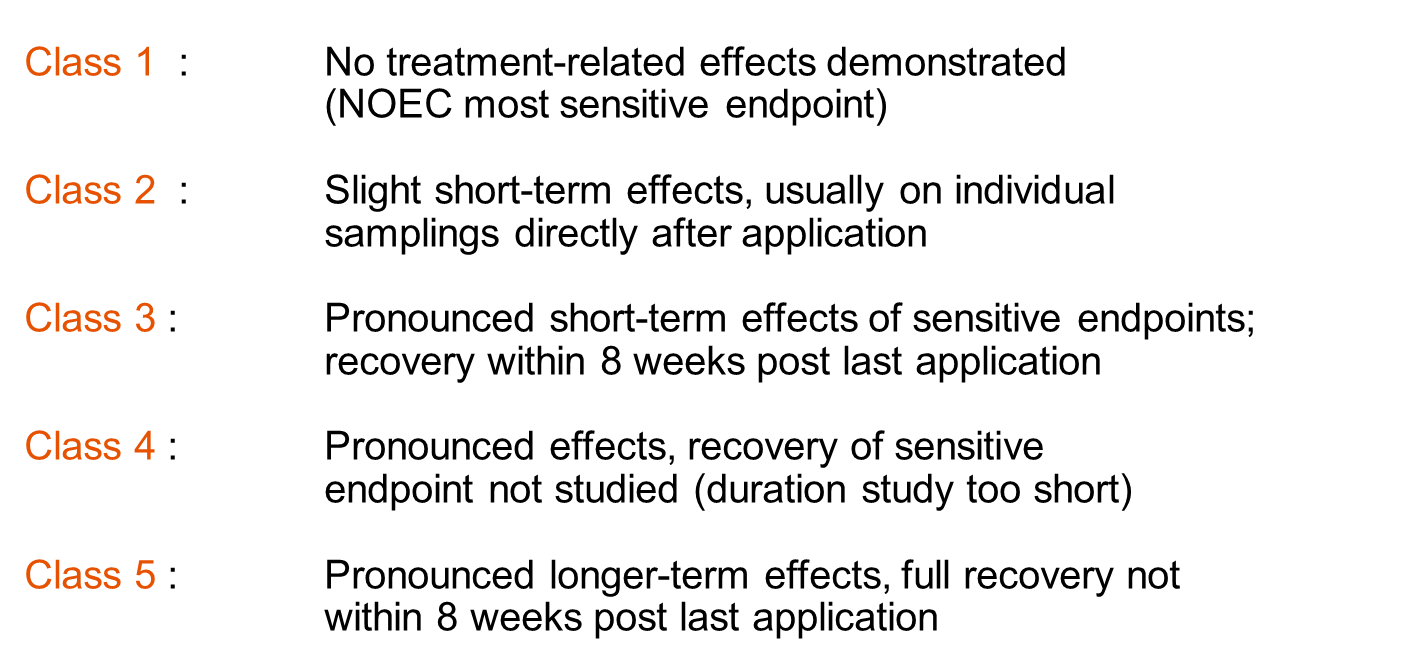
Given the large scale and complexity of experiments at the community level, such experiments generally generate overwhelming amounts of data, making appropriate analysis of the results challenging. Data analysis focusing on individual responses, so-called univariate analysis, that suffice in single species experiments, obviously falls short in community ecotoxicology, where cosm or (semi-)field communities sometimes consist of over a hundred different species. Hence, multivariate analysis is often more appropriate, similar to the approaches frequently applied in field studies to identify possible drivers of patterns in species abundances. Alternative approaches are also applied, like using ecological indices such as species richness or categorizing the responses of communities into effect classes (Figure 4). To determine if species under semi-field conditions respond equally sensitive to toxicant exposure as in the laboratory, the construction and subsequent comparison of species sensitivity distributions (SSD) (see section on SSDs) may be helpful.
The analysis and interpretation of community ecotoxicity data is also challenged by the dynamic development of each individual replicate cosm, artificial pond, ditch or stream, including those from the control. From the start of the experiment, each control replicate develops independently, matures, and at the end of the experiments that generally last for several months control replicates may differ not only from the treatments, but also among each other. The challenge is then to separate the toxic signal from the natural variability in the data.
In experiments that include a recovery phase, it is frequently observed that previously exposed communities do recover, but develop in another direction than the controls, which actually challenges the definition of recovery. Moreover, recovery can be decelerated or accelerated depending on the dispersal capacity of the species potentially inhabiting the cosms and the distance to nearby populations within a metapopulation (see section on Metapopulations). Other crucial factors that may affect the impact of a toxicant on communities, as well as their recovery from this toxicant exposure include habitat heterogeneity and the state of the community in combination with the moment of exposure. Habitat heterogeneity may affect the distribution of toxicants over the different environmental compartments and may provide shelter to organisms. Communities generally exhibit temporal dynamics in species composition and in their contribution to ecosystem processes (see section on Structure versus function), as well in the lifecycle stages of the individual species. Exponentially growing populations recover much faster than populations that reached carrying capacity and for almost all species, young individuals are up to several orders of magnitude more sensitive than adults or late instar larvae (see section on Population ecotoxicology). Hence, the timing of exposure to toxicants may seriously affect the extent of the adverse effects, as well as the recovery potential of the exposed communities.
From community ecotoxicology towards ecosystems and landscapes
When scaling up from the community to the ecosystem level, again unique characteristics emerge: structural characteristics like biodiversity, but also ecosystem processes, quantified by functional endpoints like primary production, ecosystem respiration, nutrient cycling and decomposition. Although a good environmental quality is based on both ecosystem structure and functioning, there is definitely a bias towards ecosystem structure, both in science and in policy (see section on Structure versus function). Levels of biological organisation higher than ecosystems are covered by the field of landscape ecotoxicology (see section on Landscape ecotoxicology) and in a more practical way by the concept of ecosystem services (see section on Ecosystem services).
References
Roessink, I., Crum, S.J.H., Bransen, F., Van Leeuwen, E., Van Kerkum, F., Koelmans, A.A., Brock, T.C.M. (2006). Impact of triphenyltin acetate in microcosms simulating floodplain lakes. I. Influence of sediment quality. Ecotoxicology 15, 267-293.
Roessink, I., Arts, G.H.P., Belgers, J.D.M., Bransen, F., Maund, S.J., Brock, T.C.M. (2005). Effects of lambda-cyhalothrin in two ditch mesocosm systems of different trophic status. Environmental Toxicology and Chemistry 24, 1684-1696.
Further reading
Clements, W.H., Newman, M.C. (2002). Community Ecotoxicology. John Wiley & Sons, Ltd.
Motivate the importance of studying ecotoxicology at the community level.
Define community ecotoxicology and name specific phenomena at the community and ecosystem level.
Roessink et al. (2006) studied the impact of the fungicide triphenyltin acetate (TPT) on benthic communities in outdoor mesocosms. For several species they observed a dose related decrease in abundance directly after application, followed by a gradual recovery, see example in the graph below.
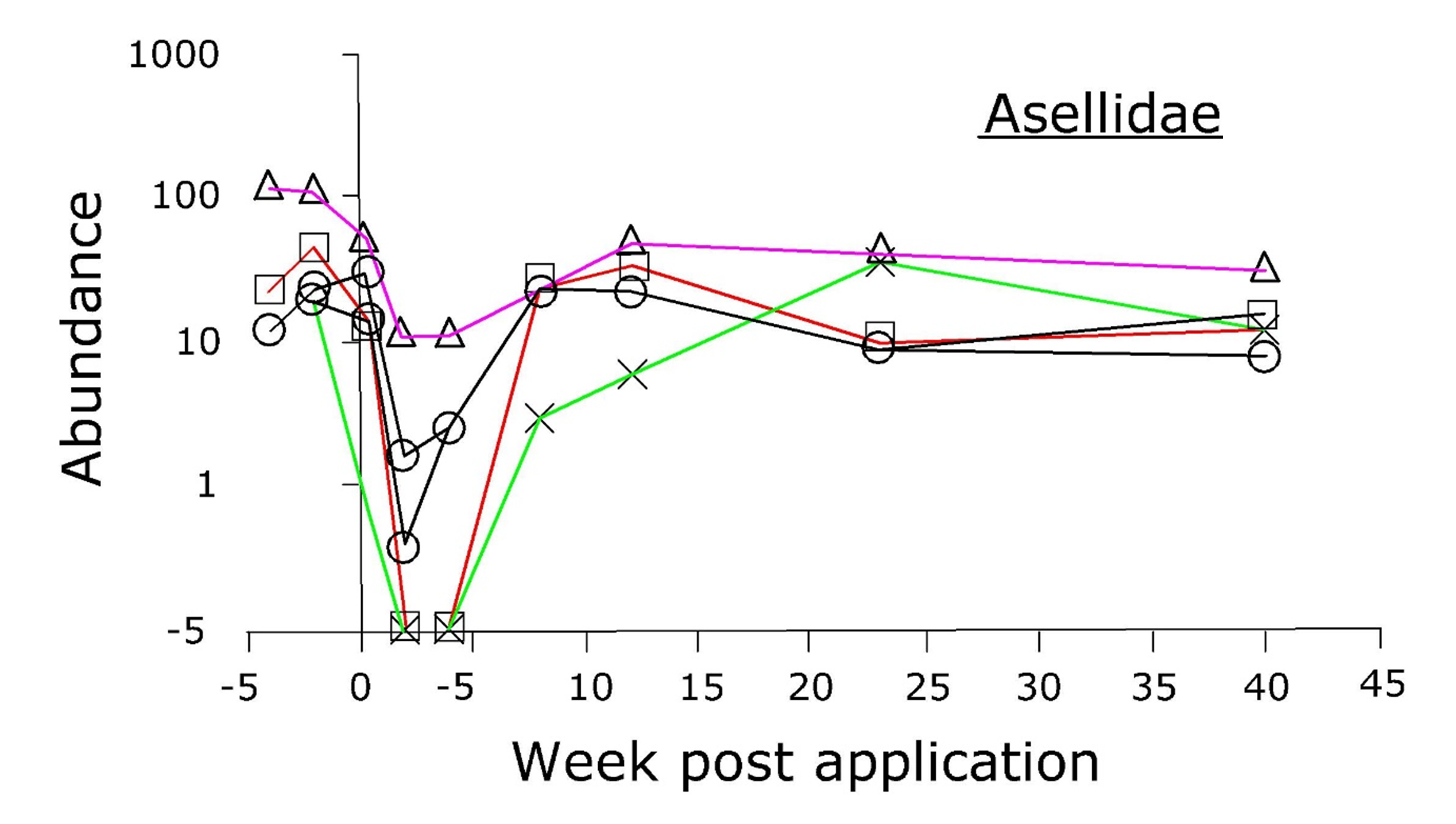
For some species however, opposite results were obtained and the abundance increased shortly after application, followed by a gradual decline, see example in the graph below.

Explain the results shown in the lower graph.
Mention three ways to analyze data from experiments at the community level.
Mention one advantage and three disadvantages of cosm experiments.
5.7.2. Community ecotoxicology in practice
Author: Martina G. Vijver
Reviewers: Paul J. van den Brink, Kees van Gestel
Learning objectives:
To be able to
- describe the variety of ecotoxicological test systems available to address different research questions.
- explain what type of information is gained from low as well as higher level ecotoxicological tests.
- explain the advantages and disadvantages of different higher level ecotoxicological test systems
Keywords: microcosms, mesocosms, realism, different biological levels
Introduction: Linking effects at different levels of biological organization
It is generally anticipated that ecotoxicological tests should provide data useful for making realistic predictions of the fate and effects of chemicals in natural ecosystems (Landner et al., 1989). The ecotoxicological test, if used in an appropriate way, should be able to identify the potential environmental impact of a chemical before it has caused any damage to the ecosystem. In spite of the considerable amount of work devoted to this problem and the plethora of test methods being published, there is still reason to question whether current procedures for testing and assessing the hazard of chemicals in the environment do answer the questions we have asked. Most biologists agree that at each succeeding level of biological organization new properties appear that would not have been evident even by the most intense and careful examination of lower levels of organization (Cairns Jr., 1983).
These levels of biological hierarchy might be crudely characterized as subcellular, cellular, organ, organism, population, multispecies, community, and ecosystem (Figure 1). At the lower biological level, responses are faster than those occurring at higher levels of organization.
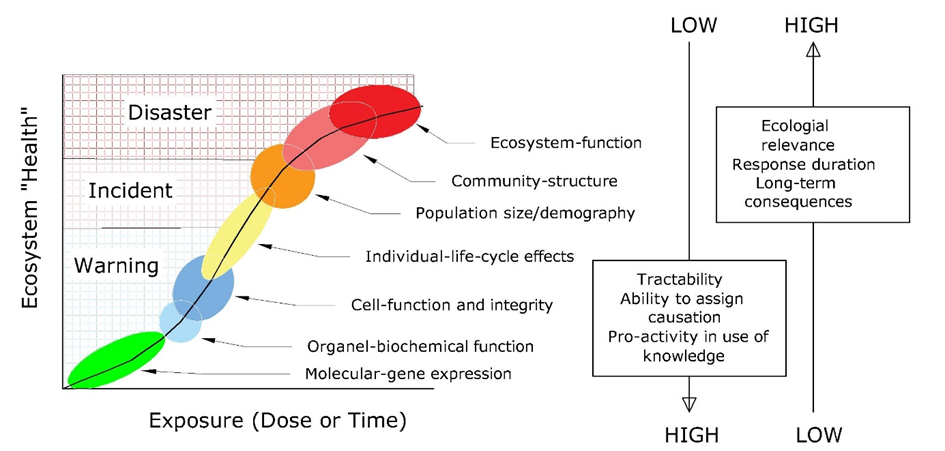
Experiments executed at the lower biological level often are performed under standard laboratory conditions (see Section on Toxicity testing). The laboratory setting has advantages like allowing for replication, the use of relatively easy and simplified conditions that enable outcomes that are rather robust across different laboratories, the stressor of interest being more traceable under optimal stable conditions, and easy repetition of experiments. As a consequence, at the lower biological level the responses of organisms to chemical stressors tend to be more tractable, or more causal, than those identified when studying effects at higher tiered levels.
The merit to perform cosm studies, so at the higher biological level (see Figure 1), is to investigate the impact of a stressor on a variety of species, all having interactions with each other. This enables detecting both direct and indirect effects on the structure of species assemblages due to the chemicals. Indirect effects can become manifest as disruptions of species interactions, e.g. competition, predator-prey interactions and the like. A second important reason for conducting cosm studies is that abiotic interactions at the level of the ecosystem can be accounted for, allowing for measurement of effects of chemicals under more environmentally realistic exposure conditions. Conditions that likely influence the fate and behavior of chemical are sorption to sediments and plants, photolysis, changes in pH (see section on Bioavailability for a more detailed description), and other natural fluctuations.
What are cosm studies?
Microcosm or mesocosm (or cosm) studies represent a bridge between the laboratory and the natural world (examples of aquatic cosms are given in Figure 2). The difference between micro- and mesocosms is mostly restricted to size (Cooper and Barmuta, 1993). Aquatic microcosms are 10-3 to 10 m3 in size, while mesocosms are 10 to 104 m3 or even larger equivalent to whole or natural systems. The originality of cosms is mainly based on the combination of ecological realism, achieved by the introduction of the basic components of natural ecosystems, and facilitated access to a number of physicochemical, biological, and toxicological parameters that can be controlled to some extent. The cosm approach also makes it possible to work with treatments that can be replicated, so enabling the study of multiple environmental factors which can be manipulated. The system allows the establishment of food webs, the assessment of direct and indirect effects, and the evaluation of effects of contamination on multiple trophic and taxonomic levels in an ecologically relevant context. Cosm studies make it possible to assess effects of contaminants by looking at the parts (individuals, populations, communities) and the whole (ecosystems) simultaneously.

As given in the OECD guidance document (OECD, 2004), the size to be selected for a meso- or microcosm study will depend on the objectives of the study and the type of ecosystem that is to be simulated. In general, studies in smaller systems are more suitable for short-term studies of up to three to six months and studies with smaller organisms (e.g. planktonic species). Larger systems are more appropriate for long-term studies (e.g. 6 months or longer). Numerous ecosystem-level manipulations have been conducted since the early 1970s (Hurlbert et al., 1972). The Experimental Lakes Area (ELA) situated in Ontario, Canada deserves special attention because of its significant contributions to the understanding of how natural communities respond to chemical stressors. This ELA consists of 46 natural, relatively undisturbed lakes, which were designated specially for ecosystem-level research. Many different questions have been tackled here, e.g. manipulations with nutrients (amongst others Levine and Schindler, 1999), synthetic estrogens (e.g. Kidd et al., 2014) and Wallace with pesticides in the Coweeta district (Wallace et al., 1999). It is nowadays realized that there is a need for testing more than just individual species and to take into account ecosystem elements such as fluctuations of abiotic conditions and biotic interactions when trying to understand the ecological effects of chemicals. Therefore a selection of study parameters is often considered as given by OECD (2004):
- Regarding treatment regime:
- dosing regime, duration, frequency, loading rates, preparation of application
- solutions, application of test substance, etc.;
- meteorological records for outdoor cosms;
- physicochemical water parameters (temperature, oxygen saturation, pH, etc.);
- Regarding biological levels it should be recorded what sampling methods and taxonomic identification methods are used;
- phytoplankton: chlorophyll-a; total cell density; abundance of individual dominant taxa; taxa (preferably species) richness, biomass;
- periphyton: chlorophyll-a; total cell density; density of dominant species; species richness, biomass;
- zooplankton: total density per unit volume; total density of dominant orders (Cladocera, Rotifera and Copepoda); species abundance; taxa richness, biomass;
- macrophytes: biomass, species composition and % surface covering of individual plants;
- emergent insects: total number emerging per unit of time; abundance of individual dominant taxa; taxa richness; biomass; density; life stages;
- benthic macroinvertebrates: total density per unit area; species richness, abundance of individual dominant species; life stages;
- fish: total biomass at test termination; individual fish weights and lengths for adults or marked juveniles; condition index; general behaviour; gross pathology; fecundity, if necessary.
Two typical examples of results obtained in an aquatic cosm study
A cosm approach assist in identifying and quantifying direct as well as indirect effects. Here two different types of responses are described, for more examples it is referred to the Section on Multistress.
Joint interactions: Barmentlo et al. (2018) used an outdoor mesocosm system consisting of 65 L ponds. Using a full factorial design, they investigated the population responses of macroinvertebrate species assemblages exposed for 35 days to environmentally relevant concentrations of three commonly used agrochemicals (imidacloprid, terbuthylazine, and NPK fertilizers). A detrivorous food chain as well as an algal-driven food chain were inoculated into the cosms. At environmentally realistic concentrations of binary mixtures, the species responses could be predicted based on concentration addition (see Section on Mixture toxicity). Overall, the effects of trinary mixtures were much more variable and counterintuitive. This was nicely illustrated by how the mayfly Cloeon dipterum reacted to the various combinations of the pesticides. Compared to single substance exposures and binary mixtures, extreme low recovery of C. dipterum (3.6% of control recovery for both mixtures) was seen. However, after exposure to the trinary mixture, recovery of C. dipterum no longer deviated from the control, and therefore was was higher than expected. Unexpected effects of the mixtures were also obtained for both zooplankton species (Daphnia magna and Cyclops sp.) As expected, the abundance of both zooplankton species was positively affected by nutrient applications, but pesticide addition did not lower their recovery. These type of unexpected results can only been identified when multiple species and multiple stressors are tested and cannot be detected in a lab-test with single species.
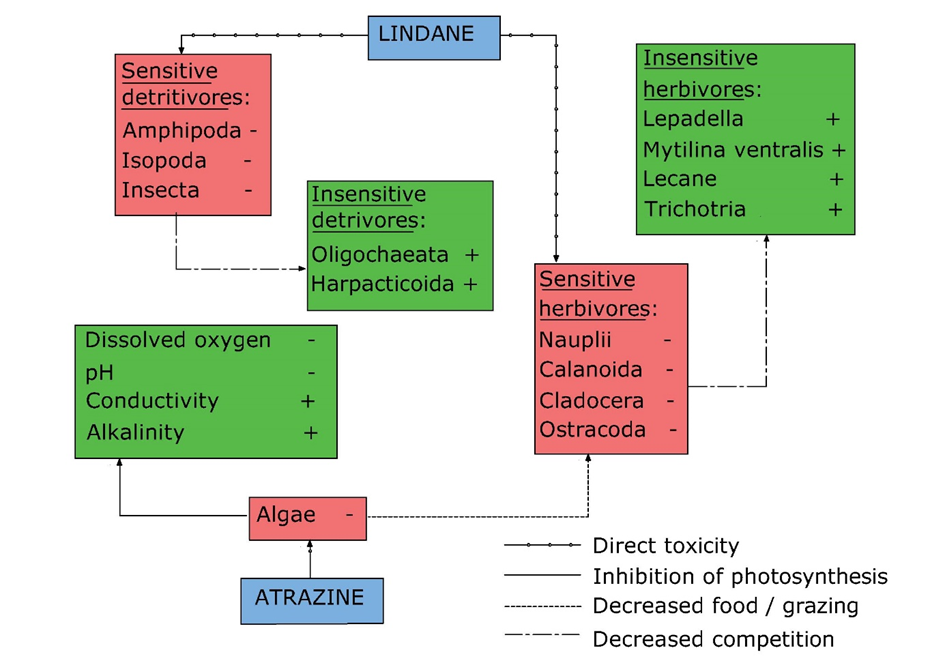
Indirect cascading effects: Van den Brink et al. (2009) studied the effects of chronic applications of a mixture of the herbicide atrazine and the insecticide lindane in indoor freshwater plankton-dominated microcosms. Both top-down and bottom-up regulation mechanisms of the species assemblage selected were affected by the pesticide mixture. Lindane exposure also caused a decrease in sensitive detritivorous macro-arthropods and herbivore arthropods. This allowed insensitive food competitors like worms, rotifers and snails to increase in abundance (although not always significantly). Atrazine inhibited algal growth and hence also affected the herbivores. A direct result of the inhibition of photosynthesis by atrazine exposure were lower dissolved oxygen and pH levels and an increase in alkalinity, nitrogen and electrical conductivity. See Figure 3 for a synthesis of all interactions observed in the study of Van den Brink et al. (2009).
Realism of cosm studies
There is a conceptual conflict between realism and replicability when applied to mesocosms. Replicability may be achieved, in part, by a relative simplification of the system. The crucial point in designing a model system may not be to maximize the realism, but rather to make sure that ecologically relevant information can be obtained. Reliability of information on ecotoxicological effects of chemicals tested in mesocosms closely depends on the representativeness of biological processes or structures that are likely to be affected. This means that within cosms key features at both structural and functional levels should be preserved as they ensure ecological representativeness. Extrapolation from small experimental systems to the real world seems generally more problematic than the use of larger systems in which more complex interactions can be studied experimentally as well. For that reason, Caquet et al. (2000) claim that testing chemicals using mesocosms refines the classical methods of ecotoxicological risk assessment because they provide conditions for a better understanding of environmentally relevant effects of chemicals.
References
Barmentlo S.H., Schrama M., Hunting E.R., Heutink R., Van Bodegom P.M., De Snoo G.R., Vijver M.G. (2018). Assessing combined impacts of agrochemicals: Aquatic macroinvertebrate population responses in outdoor mesocosms, Science of the Total Environment 631-632, 341-347.
Caquet, T., Lagadic, L., Sheffield, S.R. (2000) Mesocosm in ecotoxicology: outdoor aquatic systems. Reviews of Environmental Contamination and Toxicology 165, 1-38.
Cairns Jr. J. (1983). Are single species toxicity tests alone adequate for estimating environmental hazard? Hydrobiologica 100, 47-57.
Cooper, S.D., Barmuta, L.A. (1993) Field experiments in biomonitoring. In Rosenberg, D.M., Resh, V.H. (Eds.) Freshwater Biomonitoring and Benthic Macroinvertebrates. Chapman and Hall, New York, pp. 399-441.
OECD (2004). Draft Guidance Document on Simulated Freshwater Lentic Field Tests (Outdoor Microcosms and Mesocosms) (July 2004). Organization for Economic Cooperation and Development, Paris. http://www.oecd.org/fr/securitechimique/essais/32612239.pdf
Hurlbert, S.H., Mulla, M.S., Willson, H.R. (1972) Effects of an organophosphorus insecticide on the phytoplankton, zooplankton, and insect populations of fresh-water ponds. Ecological Monographs 42, 269-299.
Kidd, K.A., Paterson, M.J., Rennie, M.D., Podemski, C.L., Findlay, D.L., Blanchfield, P.J., Liber, K. (2014). Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen. Philosophical Transactions of the Royal Society B Biological Sciences 369, Article AR 20130578, DOI:10.1098/rstb.2013.0578
Landner, L., Blanck, H., Heyman, U., Lundgren, A., Notini, M., Rosemarin, A., Sundelin, B. (1989) Community Testing, Microcosm and Mesocosm Experiments: Ecotoxicological Tools with High Ecological Realism. Chemicals in the Aquatic Environment. Springer, pp. 216-254.
Levine, S.N., Schindler, D.W. (1999). Influence of nitrogen to phosphorus supply ratios and physicochemical conditions on cyanobacteria and phytoplankton species composition in the Experimental Lakes Area, Canada. Canadian Journal of Fisheries and Aquatic Sciences 56, 451-466.
Newman, M.C. (2008). Ecotoxicology: The History and Present Directions. In Jørgensen, S.E., Fath, B.D. (Eds.), Ecotoxicology. Vol. 2 of Encyclopedia of Ecology, 5 vols. Oxford: Elsevier, pp.1195-1201.
Van den Brink, P.J., Crum, S.J.H., Gylstra, R., Bransen, F., Cuppen, J.G.M., Brock, (2009). Effects of a herbicide - insecticide mixture in freshwater microcosms: risk assessment and ecological effect chain. Environmental Pollution 157, 237-249.
Wallace, J.B., Grubaugh, J.W., Whiles, M.R. (1996). Biotic indices and stream ecosystem processes: Results from an experimental study. Ecological Applications 6, 140-151.
Responses of organisms to long-term exposure can be detected at the sub-organism level by making use of biomarkers and then extrapolating these results to organism fitness and consequence at the population level. Mention two benefits of performing tests making use of biomarkers
Mention at least two benefits of performing tests at the higher biological level such as community or ecosystem levels.


