Cobalamin 1
- Page ID
- 500
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
Cobalamin or Vitamin B12, a water soluble vitamin that has known functions for improving brain and nerve cells, and the production of adequate blood cells.1 It is commonly found in meats, poultry, dairy products, eggs and seafood. However, organisms such as bacteria and algae are also known to produce the active form of vitamin B12 through fermentation. The structure of cobalamin is unique with the central atom; cobalt, that has potential for metalloenzyme active sites. In particular, coenzyme B12 or adenosylcobalamin (AdoCbl) is an essential for several enzymes such as methylmaonly-CoA mutase, diol dehydratase, and ethanolamine ammonia lyase.2
Cobalamin is an important biologically active, though small, enzyme involved in several configurational changes on its active site. As the name suggests, Cobalamin incorporates several structural elements surrounding a cobalt atom as the metalloenzyme active site. The vitamin-B12 configuration of Cobalamin that is of importance to biological life, primarily in the function of mechanisms in the liver, is that of Cyanocobalamin, one of the rare cases where a cyanide group is presently ingested by living organisms where otherwise would be toxic. Four main forms of Cobalamin exist and are interchanged in the body as the B12 performs various functions. The Cyanocobalamin used as the B12 vitamin supplement however is not naturally occurring, and must be generated then ingested in order to configurationally lose its cyanide group for a methyl group. Various functions in cell metabolism are involved with the transfer of the removable ligand group on the Cobalamin active site, most importantly is the transfer of methyl groups thus acting as a catalyst between configurational changes in many enzymes.
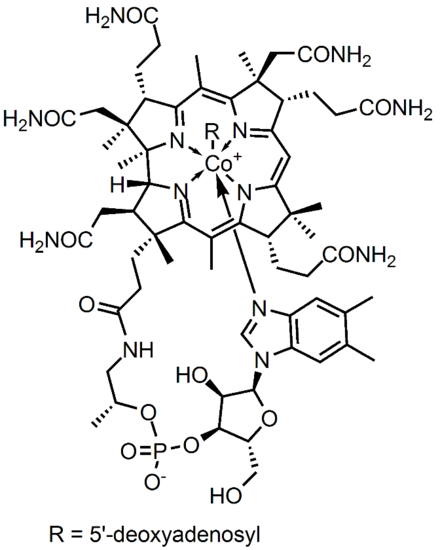
Figure 1. Adenosylcobalamin
Symmetry
Cobalamin has a formula C63H88CoN14o14P and molecular mass 1355.37 g/mol. This is a fairly large molecule, thus, analyzing the structure confirmation can determine the point group. The structure contains sigma vertical planes and has no sigma horizontal plane. The central atom, Cobalt, has an R attached that makes the molecule unique in several enzymatic catalyses. The point group of Cobalamin is assigned as C4v.
Mechanism
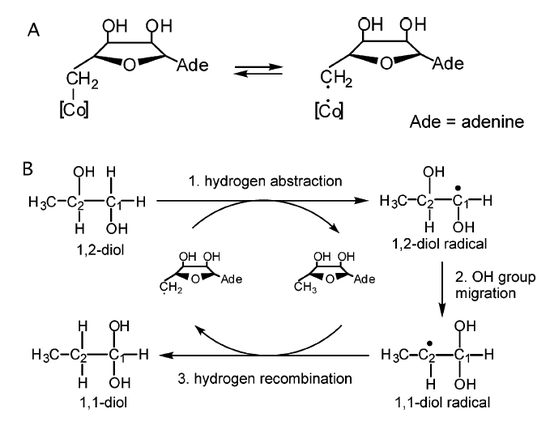
Figure 2. Minimal mechanism of diol dehydratase reaction.
The mechanism of diol dehydrates is known to catalyzes the conversion of 1,2-diols to the corresponding aldehydes. Figure 2 shows a mechanism for this enzymatic dehydration that involves the hydrogen atom abstraction from C1 (1,2-diol) and the migration of an OH group from C2 to C1 of 1,2-propanediol. The adenosyl (AdoCH2) radical that is generated by the hemolytic cleavage of the Co-C covalent bond in AdoCbl plays an essential role in this OH group migration and thus effectively promotes this chemically difficult reaction.2 Therefore, the OH group on C2 migrates to C1 leading to a formation of a 1,1-diol radical, which leads to the formation of the 1-1-diol and the regeneration of AdoCH2 radical.
The crystal structure of diol dehydratase with cyanocobalamin and adeninlypentylcobalamin have shown that both the OH groups of substrate coordinate directly to K+ ion at the active site, which implies the participation of K+ ion in the OH group migration.3 Kamachi and colleagues performed density functional theory to reveal the catalytic roles of K+ ion in the diol dehydratase reaction. As a result, the course of a reaction the substrate and the radical intermediates are always bound to K+ ion until the release of product aldehyde from the active site and that OH group proceed with the aid of K+. Therefore, the role of K+ ion have suggest that it is the most important role in the reaction to fix the substrate and the intermediates in a proper position in order to ensure the hydrogen abstraction and recombination.
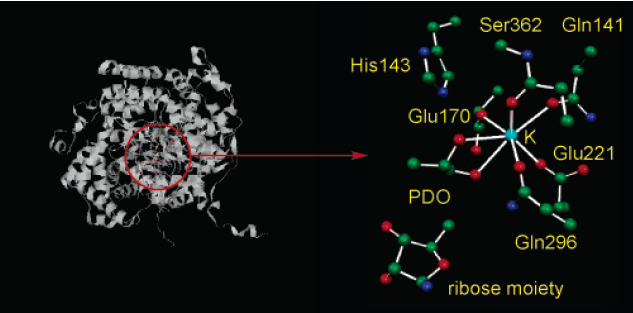
Figure 2 shows the optimized structure of the enzyme in the QM region. K+ ion is corresponding to the five oxygen atoms originated from the side chain of Gln141, Glu170, Glu221, Gln296, and the carbonyl group of Ser352.2 The sixth and the seventh coordination positions are occupied by O1 and O2 of the substrates (S)-1,2-propanediol (PDO); the S-enantiomer is preferred in the binding by enzyme.2 The ribose moiety of 5’-deoxyadenosyl radical and the side chain of His143 are also involved in the QM region.
The interaction of the migrating OH group with the imidazolum ion of His143 has been considered to be essential for the stabilization of the transition state for the OH migration. The Ribosyl rotation for the radical transfer from AdoCbl to substrate can essentially promote the Co-C cleavage upon binding to apodiol dehydratase, where adeninylpropylcobalamin (AdePeCbl) and other longer chain homologues cannot.2 The presence of the adenine-binding site in dio dehydratase was recently determined by the crystal structure analysis of the diol hydratase-AdePeCbl complex.8 The crystal structure shows that the adenine moiety of this analogue is trapped by hydrogen-bonding network with a water molecule and surrounding amion acid residues, Ser224, Ser229, Ser301, and Gly261.2 For this reason, the adenine-binding pocket fixes the adenine ring to allow tight binding of adenylpentyl group to the Co atom at a distance of 1.89 A, which is the main reason for the catalytic inactivity of the analogue.
[NEED TO RELOAD THIS IMAGE PROPERLY]
Figure 4. Active site structure of diol dehydratase.
Figure 4, part A shows an X-ray structure of the diol dehydratase-AdePeCbl complex. Part B shows Diol dehydratase-AdoCbl model complex produced by replacing the pentyl moiety of A with ribose. Part C shows the optimized structure of the diol dehydratase- AdoCbl complex model after the rotation of the ribose moiety.4 However, there is still argument whether the K+ ion in the active site remains with the substrate and radical intermediates through the reaction. Further studies could contribute to the understanding of hydrogen bonds to the active site residues, hydrogen abstraction and the steroselective hydrogen recombination.
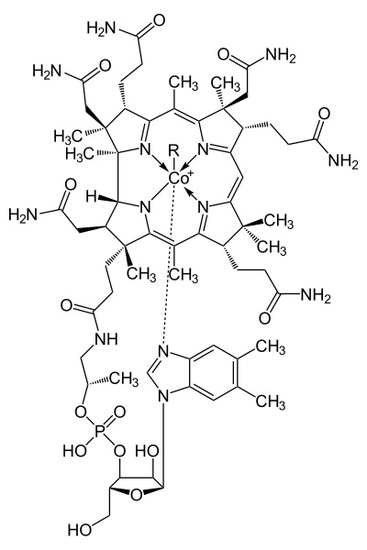
Where R = -OH, Hydroxycobalamin, -CN, Cyanocobalamin, -Me, Methylcobalamin, -Ado, 5-deoxyadensosine. The Cyanocobalamin is generated in-situ by bacteria in the gastrointestinal systems of many mammals, or by the carbonization of Hydroxycobalamin created by other types of bacteria when exposed to a charcoal environment. While the B12 ingested may be the Cyanocobalamin, the cyanide group is removed in the body when absorbed and is decomposed in the process of removal. Once in the body, the B12 structure is used in the transport of methyl groups in DNA construction as well as 5-deoxyadensosine in mitochondrial energy production in cells. However important this B12 interaction is in the human body, humans don't naturally utilize nor produce any of this B12, rather the functional use of Folic Acid in the body is merely replaced by Cobalamin. As a result, many health effects of both deficiency of B12 or folic acid can be rectified by the addition of the other if need be. Deficiency of either B12 or folic acid can result in several neurological disorders and lack of motivation or onset of depression, which can possibly be related to the available energy production in cells being altered or slowed down due to this deficiency. However, too much Cobalamin in the human blood stream can potentially lead to several serious diseases, many effects of which are still being researched and less understood due to its homogenous behavior alongside other compounds already present in the human body, such as folic acid. Several of these diseases are both the cause of overutilization of Cobalamin, and the resulting effect of which, including several types of leukemia, resulting in the high levels of Cobalamin being stored in tissues. This large amount of stored Cobalamin being involved in the high presence of haptocorrin, from the corrin portion of the cobalamin structure, leading to several, some life threatening, liver diseases. Even though
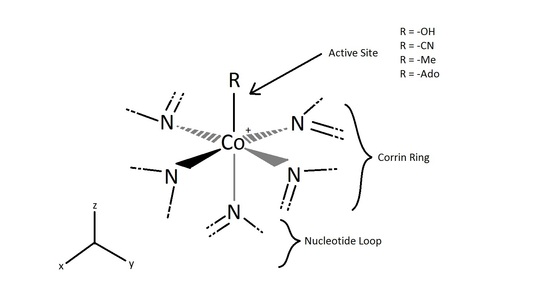
The active site of the cobalt metal in Cobalamin possesses an octahedral configuration, forming primary sigma bonds with the transfer ligand, R, and the amine ring in the Corrin ring structure, while secondary pi-type bonding occurs in the three planar imidazole-type rings of the Corrin structure, and with the imidazole-type rings below axis, as shown above. This structural configuration allows a unique electron configuration along with nearby pi-system stabilization making the cobalt atom active site a preferred and semi-stable target for transferring the hydroxyl, methyl, cyano, and 5-deoxyadensosine in the various functions of its enzyme catalysis action. If only the three planar and one sub-axial ligand bonds from the nitrogens in the imidazole-like ring structures are taken as identical, and the sigma bonding amine group and 'R' active site group are taken as separate, the cobalt and its immediate ligand environment can be seen to possibly possess a Cs type symmetry with one mirror plane running through the R and amine ligands.
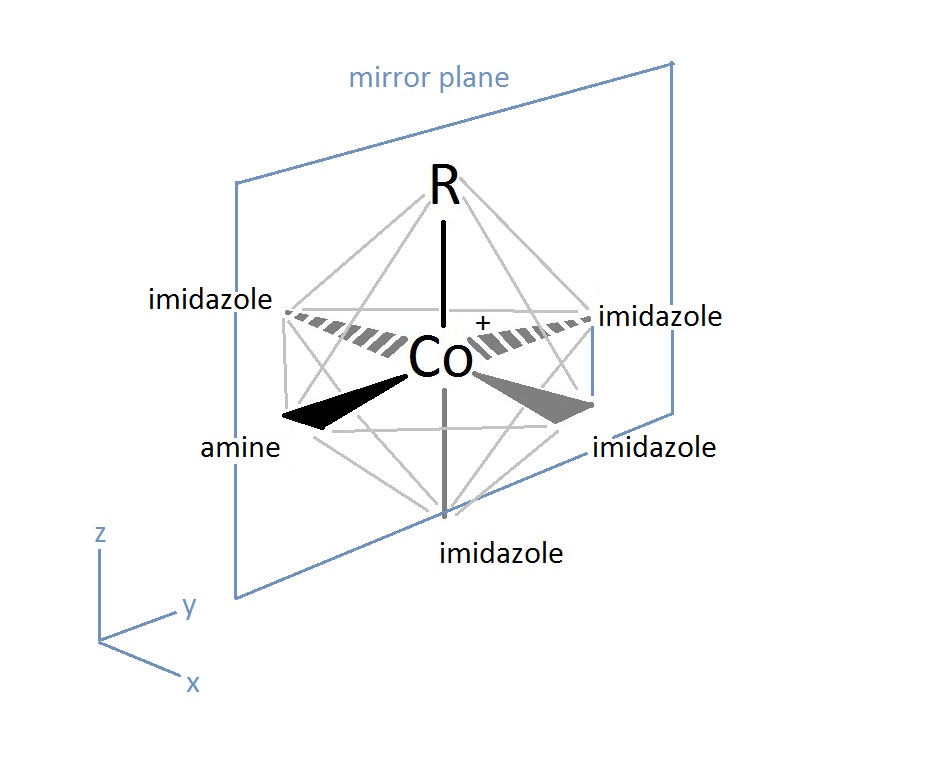
Though the above Cs symmetry applies if only the simple immediate ligand environment is considered, because of the complexity in the structure of the actual surrounding Corrin ring and Nucleotide loop in the plane and below the active site of the imidazole-type structures can't in reality be taken as identical once the structure is extended beyond a couple bond lengths away. Though this may be the case, the immediate electron density of the supposed identical imidazole-type ring groups connected to the cobalt metal may be considered near identical enough to promote the rotational configuration of the 'R' group attached to be related to the amine ligand link instead. For the R groups being -OH, -CN, or Me this has no effect whatsoever on the bonding rotation as these ligands are considered symmetric along the attachment site, however the 5-deoxyadensosine link used extensively in the energy production in cells will be effected by this symmetry of the active site, and could potentially be one reason this Cobalamin performs well in the transfer of these groups.
Cobalamin is an important compound used in the human body which is used in various configurations for specific tasks in enzymatic catalysis in subgroup transfer between systems, though not naturally utilized, it is more of a replacement for Folic Acid in the human body for these same functions, making it an important vitamin in sufficient, but not over extensive, quantities.
Reference
- “Definition of Vitamin B12” Web May 22, 2010. <http://nutrition.about.com/od/nutrientglossary/g/vitaminb12.htm>
- Kamachi, T.; Toraya, T,; Yoshizawa, K. J. Am. Chem. Soc. 2004, 126, 16207-16216
- Masuda, J.; Shibata, N.; Morimoto, Y.’ Toraya.; Yasuoka, N. Structure 2000, 8, 775.
- Eda, M.; Kamachi, T.; Yoshisawa, K.; Toraya, T. Bull. Chem. Soc. Jpn 2002, 75, 1469.
- “Figure 1” Web May 22,2010. <http://en.Wikipedia.org/wiki/Vitamin_B12>
- A. A. M. Ermens, L. T. Vlasveld, J. Lindemans, Significance of elevated cobalamin (vitamin B12) levels in bloodClinical Biochemistry, Volume 36, Issue 8, November 2003, Pages 585-590
- Harry P. C. Hogenkamp, Douglas A. Collins, David Live, Linda M. Benson, Stephen Naylor, Synthesis and characterization of nido-carborane-cobalamin conjugates. Nuclear Medicine and Biology, Volume 27, Issue 1, January 2000, Pages 89-92
- Tilak Chandra, Kenneth L. Brown, Vitamin B12 and α-ribonucleosides Tetrahedron, Volume 64, Issue 1, 1 January 2008, Pages 9-38
- Lucio Randaccio, Silvano Geremia, Jochen Wuerges, Crystallography of vitamin B12 proteins, Journal of Organometallic Chemistry, Volume 692, Issue 6, 15 February 2007, Pages 1198-1215
Contributors and Attributions
- Kris Tapper (UCD)

