Electron Transport Chain
- Page ID
- 455
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The electron transport chain (aka ETC) is a process in which the NADH and [FADH2] produced during glycolysis, β-oxidation, and other catabolic processes are oxidized thus releasing energy in the form of ATP. The mechanism by which ATP is formed in the ETC is called chemiosmotic phosphorolation.
Introduction
The byproducts of most catabolic processes are NADH and [FADH2] which are the reduced forms. Metabolic processes use NADH and [FADH2] to transport electrons in the form of hydride ions (H-). These electrons are passed from NADH or [FADH2] to membrane bound electron carriers which are then passed on to other electron carriers until they are finally given to oxygen resulting in the production of water. As electrons are passed from one electron carrier to another hydrogen ions are transported into the intermembrane space at three specific points in the chain. The transportation of hydrogen ions creates a greater concentration of hydrogen ions in the intermembrane space than in the matrix which can then be used to drive ATP Synthase and produce ATP (a high energy molecule).
Overview
In the diagram located below there are the major electron transporters responsible for making energy in the ETC.
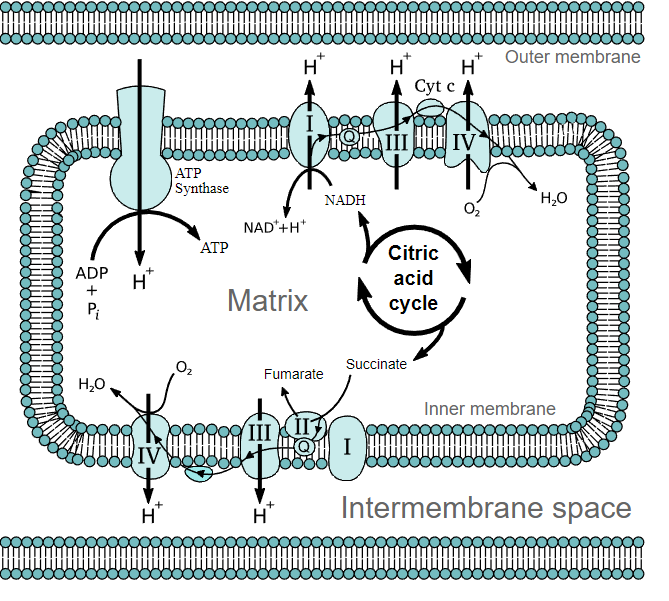
The Electron Carriers
- I (NADH-ubiquinone oxidioreductase): An integral protein that receives electrons in the form of hydride ions from NADH and passes them on to ubiquinone
- II (Succinate-ubiquinone oxidioreductase aka succinate dehydrogenase from the TCA cycle): A peripheral protein that receives electrons from succinate (an intermediate metabolite of the TCA cycle) to yield fumarate and [FADH2]. From succinate the electrons are received by [FAD] (a prosthetic group of the protein) which then become [FADH2]. The electrons are then passed off to ubiquinone.
- Q (Ubiquinone/ ubiquinol): Ubiquinone (the oxidized form of the molecule) receives electrons from several different carriers; from I, II, Glycerol-3-phosphate dehydrogenase, and ETF. It is now the reduced form (ubiquinol) which passes its electron off to III.
- III (Ubiquinol-cytochrome c oxidioreductase): An integral protein that receives electrons from ubiquinol which are then passed on to Cytochrome c
- IV (Cytochrome c oxidase):An integral protein that that receives electrons from Cytochrome c and transfers them to oxygen to produce water within the mitochondria matrix.
- ATP Synthas: An integral protein consisting of several different subunits. This protein is directly responsible for the production of ATP via chemiosmotic phosphorolation. It uses the proton gradient created by several of the other carriers in the ETC to drive a mechanical rotor. The energy from that rotor is then used to phosphorolate ADT to ATP.
Not Shown
- ETF (Electron-transferring flavoprotein) Dehydrogenase: This peripheral protein located on the matrix side of the inner membrane is a part the B-oxidation cycle. Electrons from acyl-CoA are donated to an electron-transfer flavoprotien which are then transferred to ETF (Electron-transferring flavoprotein) Dehydrogenase in the form of [FADH2]. ETF dehydrogenase then passes those electrons from [FADH2] to ubiquinone and on through the ETC.
- Glycerol-3-phosphate dehydrogenas:This peripheral protein located on the intermembrane space side of the inner membrane is a part of the glycerol-3-phosphate transport system. It accepts a proton from glycerol-3-phosphate to a prosthetic [FAD] group which yields [FADH2]. From [FADH2] the electrons are then given to ubiquinone and on through the ETC.
Electron Flow
It should be noted from the diagram below that ubiquinone (a hydrophobic carrier that resides within the membrane) receives electrons from several different electron carriers. Cytochrome c (a hydrophilic carrier found with in the intermembrane space) on the other hand only transfers electrons from III to IV. The driving force of the ETC is the fact that each electron carrier has a higher standard reduction potential than the one that it accepts electrons from. Standard reduction potential is a measure of the ability to accept or donate electrons. Oxygen has the highest (most positive) standard reduction potential which means that is is most likely to accept electrons from other carriers. That is precisely why it is found at the end of the ETC.
Proton Motive Force
Proton motive force refers to the energy obtained from the proton gradient created by several of the electron carriers. Only three of the four mentioned electron carriers are capable of transporting protons from the matrix to the intermembrane space: I, III, and IV. It is this proton gradient that drives phosphorolation of ADP to ATP as well as several other important transport systems. As proton concentration builds up in the intermembrane space a gradient is created and protons are transported from high to low concentration. The energy from the transfer of protons is used to change ADP into ATP though phosphorolation. ATP synthase is the protein responsible for ADP phosphorolation.
It is also important for proper concentrations of substrates to be maintained within and without the mitochondria to allow for chemiosmotic phosphorolation. The two main types of proteins responsible for maintaining proper substrate concentrations are pyruvate and phosphate symporters and ADP/ATP antiporters.

