1.7: Carbohydrates
- Page ID
- 306244
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fermentation Carbohydrates
Carbohydrates are the most abundant biomolecules on earth and are widely used by organisms for structural and energy-storage purposes. In particular, glucose is the most commonly used monosaccharide, thus, this is why all of the pathways that we have covered start with glucose.
However, many microorganisms are able to utilize more complex carbohydrates for energy.
Let’s look at the structures of different carbohydrates and their use in microbial metabolism.
Monosaccharides
Monosaccarides are the building blocks (monomers) for the synthesis of polymers. These sugars are classified by the length of the chain and the position of the carbonyl.
Glucose and Ribose are shown below.
- They are both aldoses because the carbonyl is a [ketone/ aldehyde].
- One is a hexose and one is a pentose. Label each.
Glyderaldehyde and dihydroxyacetone are shown below.
- One is an aldose and one is a ketone. Label each.
- They are both ______________oses
Monosaccharides of four or more carbon atoms are typically more stable when they adopt cyclic, or ring, structures. This is a nucleophilic addition the results in a hemiacetal.
- Draw arrows for this forward reaction.
- Draw arrows for the reaction back to the straight chain.
Stereochemistry of Cyclic Sugars
There hemiacetal formed when the sugar cyclizes is a new chiral center. Two possible orientations can be formed.
- Circle the new chiral center on the two possible isomers (\(\alpha\)-glucose and \(\beta\)-glucose) below. This is called the anomeric carbon.
Disaccharides
Disaccharides are carbohydrates composed of two monosaccharide units that are joined by a carbon–oxygen-carbon linkage known as a glycosidic linkage.
Three common disaccharides are the grain sugar maltose, made of two glucose molecules; the milk sugar lactose, made of a galactose and a glucose molecule; and the table sugar sucrose, made of a glucose and a fructose molecule.
- Circle the disaccharide linkage in each of these disaccharides from the table below.
| Maltose | Lactose | Sucrose |
There are different types of glycosidic linkages. They are characterized by the numbering of the alcohols that are linked in the ether. And the anomer of the sugar.
- For the maltose shown here, the sugars are [ \(\alpha / \beta\) ] anomers. Circle one.
- Which alcohols are linked? Numbering proceeds around the ring starting with the anomeric carbon.
Thus, this is alpha-1,4-maltose.
- Draw \(\beta\)-1,4-maltose, where the glucose on the right has isomerized.
- What type of glycosidic linkage is present in this lactose isomer?
- What type of glycosidic linkage is present in sucrose?
The human body is unable to metabolize maltose or any other disaccharide directly from the diet because the molecules are too large to pass through the cell membranes of the intestinal wall. Therefore, an ingested disaccharide must first be broken down by hydrolysis into its two constituent monosaccharide units. In the body, such hydrolysis reactions are catalyzed by enzymes such as maltase or lactase.
** This will be important in upcoming discussions of beer, cheese, and yogurt production!
Polysaccharides
Polysaccharides are very large polymers composed of hundreds to thousands of monosaccharides. These structures are used for energy storage or, in the case of cellulose, structural components. Starch is a mixture of two polysaccharides and is an important component of grains (wheat, rice, barley, etc.). This will again be important in bread and beer fermentations. These two polymers are amylose and amylopectin.
Amylose is a straight chain polysaccharide (shown below).
- What monomer is present?
- What type of linkage is present?
- This structure becomes a spiral. What IMF and geometry effects might cause the structure to take on this shape?
- Amylose is not water soluble even though the monosaccharides are soluble. Suggest a reason why this is not soluble in water. Consider how easily the water might be able to interact with the spiral structure.
- Amylase cleaves only internal alpha (1-4) glycosidic bonds. Which disaccharide will be formed when amylose is being hydrolyzed by amylase
Amylopectin is a branched-chain polysaccharide. (cartoon shown below)
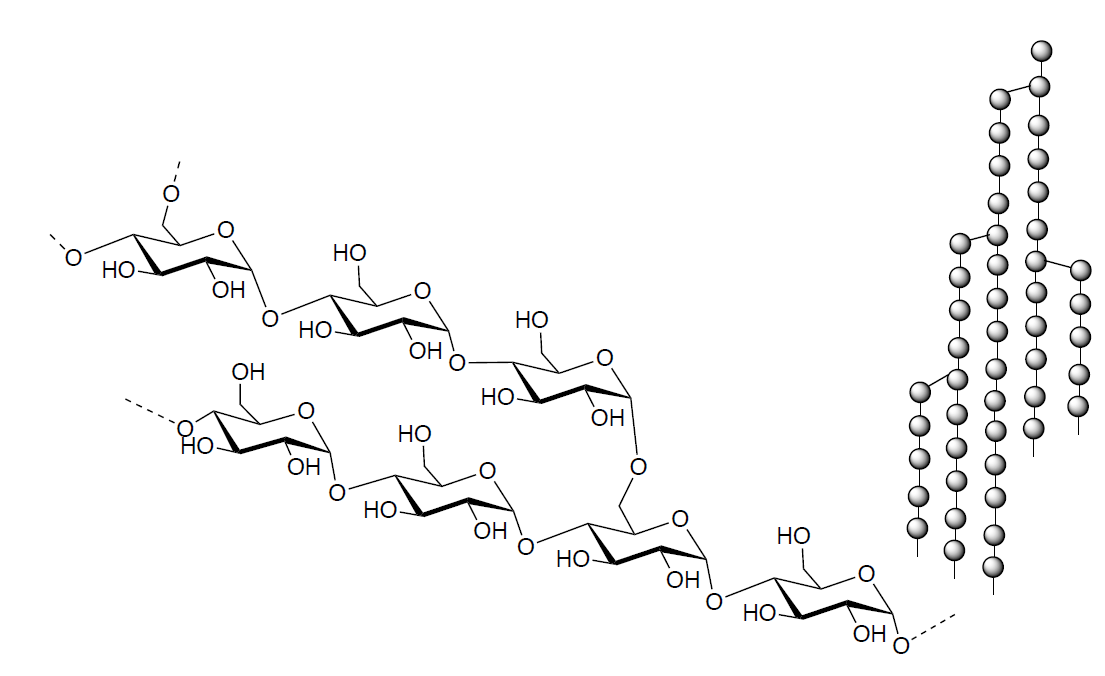
- What monomer makes up this polymer?
- There are two types of linkage are present. What are they?
- Amylopectin is water-soluble but amylose is not. Show how water might interact with amylopectin above (IMF) so that it will dissolve.
- The branched linkages are hydrolyzed by isoamylase, while the 1-4 linkages are hydrolyzed by amylase. What is the disaccharide produced?
- Amylopectin is more easily digested than the amylose. This is due to the packing of amylose. Explain.
HW questions:
Another Polysaccharide: Cellulose
- Draw cellulose.
- How does it differ from amylose?
- Cellulose is not digestible by mammals (unless they have a symbiotic bacteria in their gut). Why?


