15: Nu Sub alpha alkylation
- Page ID
- 144093
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
α-Alkylation of Ketones: Another SN2 Reaction
Review: Enols, Enolates and Enamines

NB: These topics are covered in OWL and in your R1 workbook. Use these resources to take notes here.
Keto-enol tautomerisation can occur under basic or acidic conditions.
- Draw a mechanism for the conversion of the ketone to the enol under basic conditions.

- Draw a mechanism for the conversion of the enol to the ketone under acidic conditions.
- Draw a mechanism for the conversion of iminium to the enamine under basic conditions.

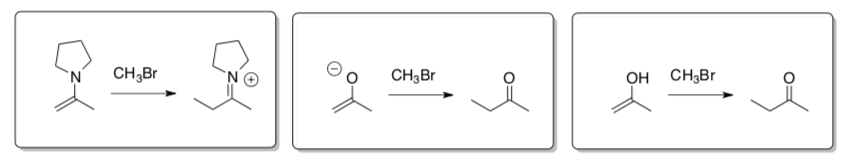
Alpha-Alkylation: Substitutions with Enolates & Enamines
Enols, Enolates and Enamines are good carbon nucleophiles. When these nucleophiles do an SN2 reaction on alkyl halide or alkyl tosylate, the reaction is called an a-alkylation.
- Draw the mechanism for each of the reactions below:

Practice Problems
- Predict the product for these reactions.
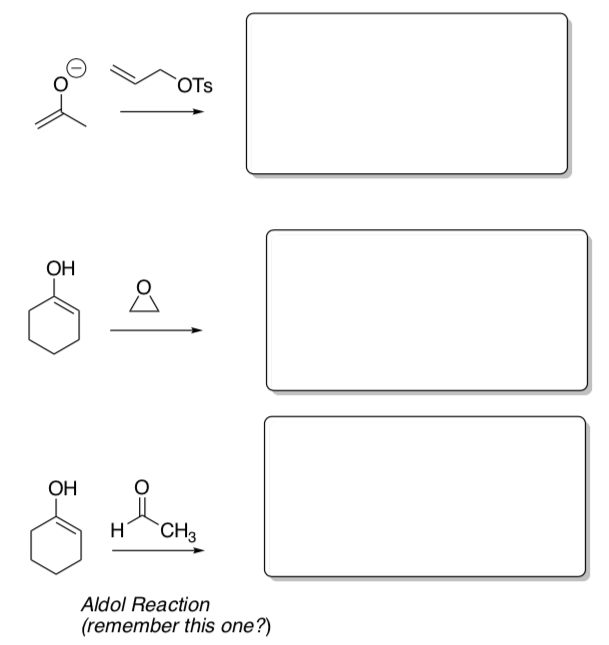
- Draw the mechanism for these reactions.

Summary of alpha alkylations
α-Alkylation with different electrophiles

- Draw the mechanism for each of the reactions below:

Practice Problems
- The following enolate alkylation has been reported by Conia (Tetrahedron, 1961,16, 45.) Provide the structure of the major product.

- The following enolate alkylation has been reported by Kim (Tetrahedron Lett.1986,27, 943). Provide the structure of the major product.

Practice Multi-step Process: Stork Enamine Synthesis

- Draw the products for this three step sequences (Stork enamine synthesis).

- Fill in the blanks on this sequence.

- Note: These two sequences have the same starting material and product. Why would you choose the Stork enamine?
Practice: Kinetics
The bromination of acetone can also be carried out in acidic conditions:

This reaction has a determined rate law of rate = k[CH3COCH3][H+]
Given below is a possible mechanism for the reaction.
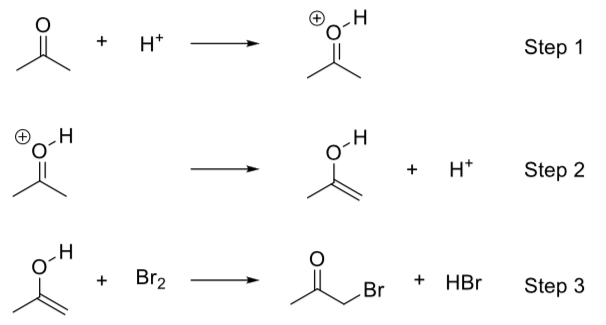
- Based on the given rate law, which step is the rate-determining step? Explain.
- Identify all catalysts and intermediates in the reaction.
Equilibrium Effects on a-Alkylation
Review: Thermodynamics of Enolate Formation
NB: These topics are covered in OWL and in your R1 workbook. Use these resources to take notes here.
Enolates are an important class of carbon nucleophiles. They are typically prepared viaa-deprotonation of carbonyl compounds, as shown below.

- Calculate ΔG for each reaction.
- Draw a representative reaction potential diagram for each reaction.
- Estimate relative concentrations of reactants and products at equilibrium.
- If a reaction is not favored by ΔG, how can Le Chatelier’s principle help?
Lego Equilibrium Activity
Adapted from Wilson, J. Chem. Ed., 1998, 75(9), 1176-1177.
Activity 1
- Assign team members roles:
R (reactants), team P (products), and a recorder - Divide up Legos: Teammember R 40 items
Teammember P none
- Teams will exchange items: For each transaction, R will hand over half of their total number while P hands over only a quarter of their items.
- Recorderwillcompletethetablebelowforeachtransaction.
| Time |
R # Items # Transferred |
P # Items # Transferred |
||
| Initial | 40 | 0 | ||
| 1st transfer | 20 | 0 | ||
| Outcome | 20 | 20 | ||
| 2nd transfer | ||||
| Outcome | ||||
| 3rd transfer | ||||
| Outcome | ||||
| 4th transfer | ||||
| Outcome | ||||
Activity 2
- Repeat activity 1 with different starting numbers.
- Divide up Legos: Teammember R 40 items Teammember P 20 items
- Teams will exchange items: For each transaction, R will hand over half of their total number while P hands over only a quarter of their items.
- Recorder will complete the table below for each transaction.
| Time |
R # Items # Transferred |
P # Items # Transferred |
||
| Initial | 40 | 0 | ||
| 1st transfer | 20 | 5 | ||
| Outcome | 25 | 35 | ||
| 2nd transfer | ||||
| Outcome | ||||
| 3rd transfer | ||||
| Outcome | ||||
| 4th transfer | ||||
| Outcome | ||||
Equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of reactants and products have no further tendency to change with time.
- Is the reaction still occurring?
- In both directions?
- Are there equal numbers of reactants/products at equilibrium?
Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and reverse reactions are generally not zero but, being equal, there are no net changes in the concentrations of the reactant and product.
- When did this happen with the Lego activity?
- In the following pictures, when does the reaction hit equilibrium?


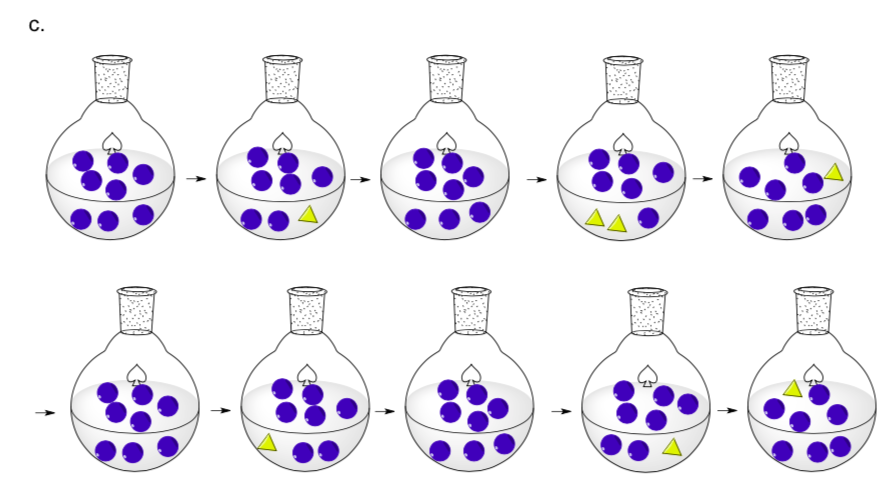
- In each case, speculate about size of ΔG?
- Ea barrier (DH‡)?
Asymmetric Carbonyl Compounds
When you start with an unsymmetrical carbonyl compound, there are two possible C nucleophiles.
- Draw the two possible enolate anion products.
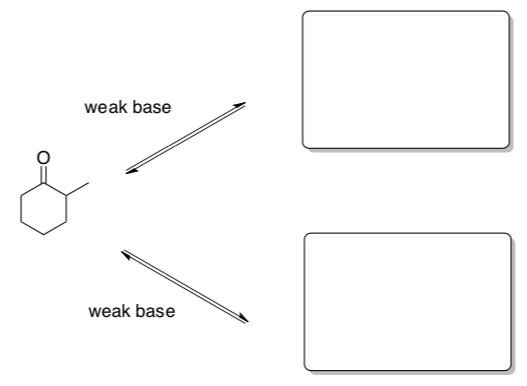
- In a weakly basic solution, these two enolates are in equilibrium. mechanism for their interconversion.
- Which enolate is more thermodynamically stable?
- Which proton is removed faster? Why?
Uh Oh... We must have two different competing reactions!
Kinetics vs Thermodynamic Pathways
In some reactions, there are two possible reaction pathways (such as in the case of deprotonating asymmetric ketones) that lead to different products.

- Which reaction has a lower Ea (DH‡)?
Dashed Solid
- Which product forms faster?
Dashed Solid
- Which reaction has a more negative DG?
Dashed Solid
- Which product is more thermodynamically stable?
Dashed Solid
- If the conditions are setup so that the reaction cannot reverse, the major product will be formed from which path?
Dashed Solid Mixed
- If the reaction is reversible and is allowed to continue until equilibrium is reached, the major product will be formed from which path?
Dashed Solid Mixed
Kinetics vs Thermodynamic Pathways: Enolate Formation

- Which reaction has a lower Ea (ΔH‡)? forms faster?
Less Substituted More Substituted
- Which reaction has a more negative DG? is more thermodynamically stable?
Less Substituted More Substituted
- Which pathway is more likely to be reversible (in dynamic equilibrium)?
Less Substituted More Substituted
- Which product is more likely to stay as product?
Less Substituted More Substituted
- Higher Temperatures will favor:
Less Substituted More Substituted
- Bulky Base (steric hindrance) will favor:
Less Substituted More Substituted
- More Ionic Character of the products will favor:
Less Substituted More Substituted
Kinetics and Thermodynamic Control of Enolate Formation
Kinetic Product Thermodynamic Product
- Temperature
- Reaction time
- Base Size (Sterics)
- Base Strength
- Ionic vs. Covalent Nature
- Counterions
- Protic vs Aprotic Solvent
Kinetic and Thermodynamic Bases
Bases Used in Enolate formation:
Here is a table of commonly used bases for enolate formation:
| Base | Conjugate Acid | pKa of Acid | % Enolate |
| NaOH | H2O | 15.7 | <1% |
| NaOEt | EtOH | 16 | <1% |
| KOtBu | tBuOH | 18 | 1-10% |
| NaH | H2 | 35 | 100% |
| LDA | HN[CH(CH3)2]2 | 40 | 100% |
- Label which base(s) would be best for formation of a kinetic enolate.
- Label which base(s) would be best for formation of a thermodynamic enolate.
Practice Problems
- Predict products for these alkylations.

- Propose a plan to synthesize these compounds from the given starting material.
Summary of Kinetic and Thermodynamic Control of alpha-Alkylations
- Draw a reaction profile for a kinetic and thermodynamic
- List some typical thermodynamic bases:
- List the most common kinetic base:
- Fill in the products for the a-alkylation of an asymmetric ketone.
- Label the enolates as ‘kinetic enolate’ or ‘thermodynamic enolate’.

- Explain why the final products in the reactions above show different regiochemical preferences.
Application Problems
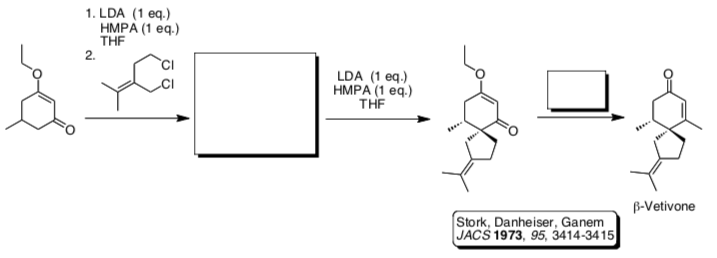
- Stork-Danheiser Alkylation
In the early 1970’s the Stork group developed an alternative route. Of particular interest, Danheiser conducted this work while an undergraduate at Columbia!
- Draw a mechanism for this reaction sequence.

- Explain the regiochemical selectivity of the alkylation.
- Complete the roadmap where Stork prepared b-Vetivone using this approach.
- Draw a mechanism for this reaction sequence.
- Muamvatin is an extraordinary octapropionate isolated from a pulmonate mollusk.
- Fill in missing reagents or products in the following synthetic procedure.
Dale Ward and Mehdi Zahedi, Org. Lett., 2012, 14(24), 6246-6249.


- Fill in missing reagents or products in the following synthetic procedure.
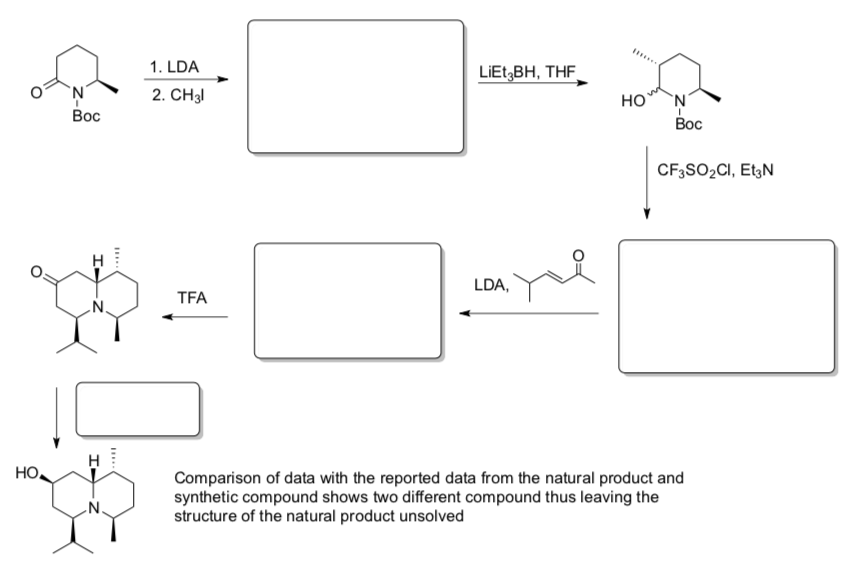
Stereoselective α-Alkylations
Alkylation on Cyclic Systems in Synthesis: Steric Control
- Draw a mechanism for the alkylation shown below:
Maldaner and Pilli, Tetrahedron, 1999, 55, 13321-13332.

- The reaction shown above is stereoselective (only one stereoisomer is produced. Looking at the intermediate shown below, draw the chair structure of the product and predict the stereochemistry (cis or trans).

- Explain why the reaction produced only one stereoisomer.
- Fill in the boxes with reagents/products for the synthesis of Plumerinine.
Maldaner and Pilli, Pure and Applied Chem., 2005, 77, 1153-1160.
The Role of Chelation in Stereoselective Alkylations
R-methylbutyramides (shown below) undergo stereospecific enolization with lithium diisopropylamide (LDA) in the presence of lithium chloride at 0 °C to form Z- and E- enolates.
Kummer, Chain, Morales, Quiroga and Myers, “Stereocontrolled Alkylative Construction of Quaternary Carbon Centers”, J. Am. Chem. Soc., 2008, 130, 13231-13233.
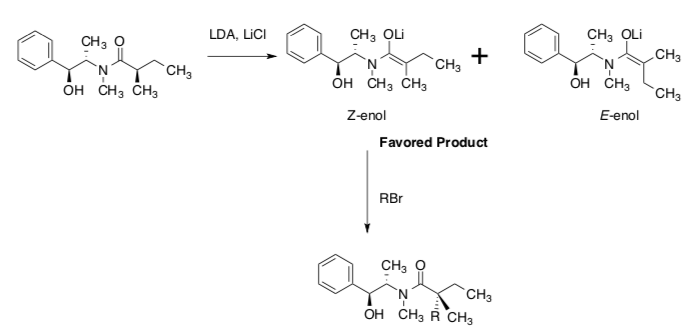
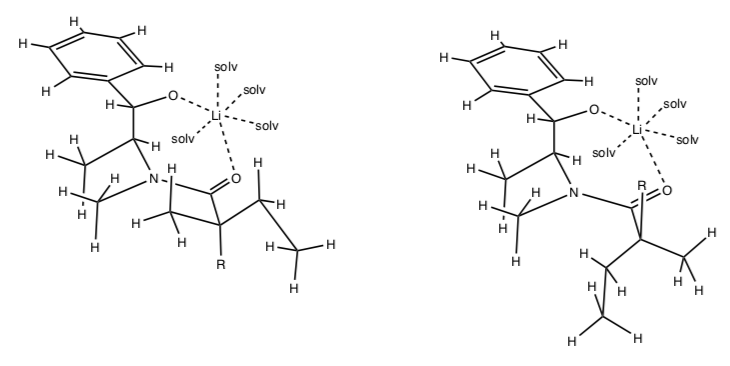
- Using the following pictures, explain why the Z-enolate is favored.
- Also explain why the subsequent stereoisomer is preferentially formed with the addition of an electrophile.
Evans Chiral Auxiliaries to Control Stereochemistry
Evans applied chiral auxiliaries to alpha-alkylation reactions, too (J. Am. Chem. Soc.1982, 104, 1737-1739).
- Show the two possible products that could be formed from this alpha-alkylation.

To address this problem, Evans attempted to use a "chiral auxiliary" – a chiral group that could be added in to the molecule to influence stereochemistry of a reaction, then removed again (J. Am. Chem. Soc. 1981, 103, 2127-2129).
- Add the appropriate reagent to add the “chiral auxiliary”.
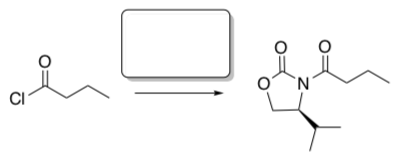
The enolate is formed to the molecule with the chiral auxiliary present.
- Draw the curved arrow mechanism for this step.

- An electrophile is added. Why does the electrophile add to only this face?

- How can the chiral auxiliary be removed?

- Show how he carried out the following transformation:

Summary of Asymmetric Alpha-Alkylations
- Regiochemistry of alpha-alkylations can be controlled by _____________.
- __________ forms the enolate that is more substituted.
- __________ forms the enolate that is less substituted.
- Stereochemistry of alpha-alkylations can be controlled by in a variety of ways:
- Maldaner and Pilli used: _____________.
- Myers used: _____________.
- Evans used: _____________.
Application Problems
- This stereoselective metalloenamine alkylation was reported by Whitesell (J. Org.Chem., 1977, 42, 377).
- Provide a mechanism for this alkylation.
- Challenge: Explain the stereochemistry. You will need to draw chelated intermediates.

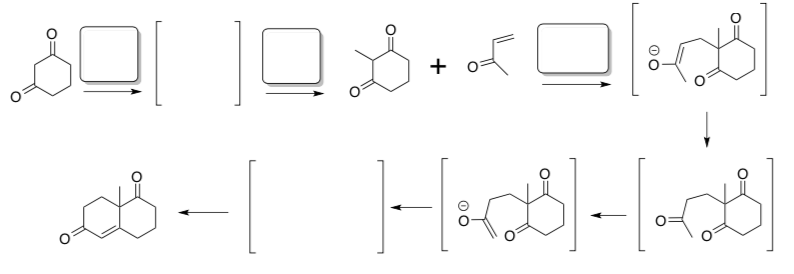
- Synthesis of Bulnesol, starting with preparation of the Wieland-Meischer ketone
The Wieland–Miescher ketone is a bicyclic ketone that is a versatile starting material utilized in the synthesis of more than 50 natural products.
The ketone was originally synthesized using the following sequence including a Robinson annulation.
- Complete the mechanism for this synthesis. Include curved arrows, missing reagents, and missing intermediates.
A historically significant enantioselective synthesis of the Wieland-Miescher ketone and the so-called Hajos-Parrish ketone proceeds via an enamine derived from L- proline. In 2004, Houk and Clemente invoked a cyclic transition state (depicted below) to account for the observed stereoselectivity
- Complete this alternative approach by adding the missing intermediate.

- What is responsible for the selectivity in this approach?
- Complete the mechanism for this synthesis. Include curved arrows, missing reagents, and missing intermediates.
- Synthesis of Bulnesol (cont.)
Ratcliffe, Heathcock, J. Am. Chem. Soc. 1971, 93, 1746.
- Fill in the missing reagents for the synthesis of bulnesol from the Wieland- Miescher ketone.

- Fill in the missing reagents for the synthesis of bulnesol from the Wieland- Miescher ketone.


