Electrospray Ionization Mass Spectrometry
- Page ID
- 320
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Electrospray ionization is a soft ionization technique that is typically used to determine the molecular weights of proteins, peptides, and other biological macromolecules. Soft ionization is a useful technique when considering biological molecules of large molecular mass, such as the aformetioned, because this process does not fragment the macromolecules into smaller charged particles, rather it turns the macromolecule being ionized into small droplets. These droplets will then be further desolvated into even smaller droplets, which creates molecules with attached protons. These protonated and desolvated molecular ions will then be passed through the mass analyzer to the detector, and the mass of the sample can be determined.
Introduction
Electrospray ionization mass spectrometry is a desorption ionization method. Desorption ionization methods can be performed on solid or liquid samples, and allows for the sample to be nonvolatile or thermally unstable. This means that ionization of samples such as proteins, peptides, olgiopeptides, and some inorganic molecules can be performed. Electrospray ionization mass spectrometry requires that a molecule be of a fairly large mass. The instrument has a small mass range that it is able to detect, so therefore the mass of the unknown injected sample can easily be determined; as it must be in the range of the instrument. This quantitative analysis is done by considering the mass to charge ratios of the various peaks in the spectrum (Figure 1). The spectrum is shown with the mass-to-charge (m/z) ratio on the x-axis, and the relative intensity (%) of each peak shown on the y-axis. Calculations to determine the unknown mass, Mr, from the spectral data can then be performed using
\[ p = \dfrac{m}{z}\]
\[p_1 = \dfrac{M_r + z_1}{ z_1} \]
\[p_2 = \dfrac{M_r + (z_1 - 1)}{z_1 - 1}\]
where p1 and p2 are adjacent peaks. Peak p1 comes before peak p2 in the spectrum, and has a lower m/z value. The z1 value represents the charge of peak one. It should be noted that as the m/z value increases, the number of protons attached to the molecular ion decreases. Figure 1 below illustrates these concepts. Electrospray ionization mass spectrometry research was pioneered by the analytical chemistry professor John Bennet Fenn, who shared the Nobel Prize in Chemistry with Koichi Tanaka in 2002 for his work on the subject.
.bmp?revision=1&size=bestfit&width=701&height=438)
Calculations for m/z in spectrum
[M + 6H]6+ [M + 5H]5+ [M + 4H]4+
m/z = 15006/6 = 2501 m/z = 15005/5 = 3001 m/z = 15004/4 = 3751
[M + 3H]3+ [M + 2H]2+ [M + H]1+
m/z = 15003/3 = 5001 m/z = 15002/2 = 7501 m/z = 15001/1= 15001
Sample Preparation
Samples for injection into the electrospray ionization mass spectrometer work the best if they are first purified. The reason purity in a sample is important is because this technique does not work well when mixtures are used as the analyte. For this reason a means of purification is often employed to inject a homogeneous sample into the capillary needle. High performance liquid chromatography, Capillary Electrophoresis, and Liquid-Solid Column Chromatography are methods of choice for this purpose. The chosen purification method is then attached to the capillary needle, and the sample can be introduced directly.
Advantages and Disadvantages
There are some clear advantages to using electrospray ionization mass spectrometry as an analytical method. One advantage is its ability to handle samples that have large masses. Another advantage is that this ionization method is one of the softest ionization methods available, therefore it has the ability to analyze biological samples that are defined by non-covalent interactions. A quadrupole mass analyzer can also be used for this method, which means that a sample's structure can be determined fairly easily. The m/z ratio range of the quadrupole instrument is fairly small, which means that the mass of the sample can be determined to with a high amount of accuracy. Finally, the sensitivity for this instrument is impressive and therefore can be useful in accurate quantitative and qualitative measurements.
Some disadvantages to electrospray ionization mass spectrometry are present as well. A major disadvantage is that this technique cannot analyze mixtures very well, and when forced to do so, the results are unreliable. The apparatus is also very difficult to clean and has a tendency to become overly contaminated with residues from previous experiments. Finally, the multiple charges that are attached to the molecular ions can make for confusing spectral data. This confusion is further fueled by use of a mixed sample, which is yet another reason why mixtures should be avoided when using an electrospray ionization mass spectrometer.
Apparatus
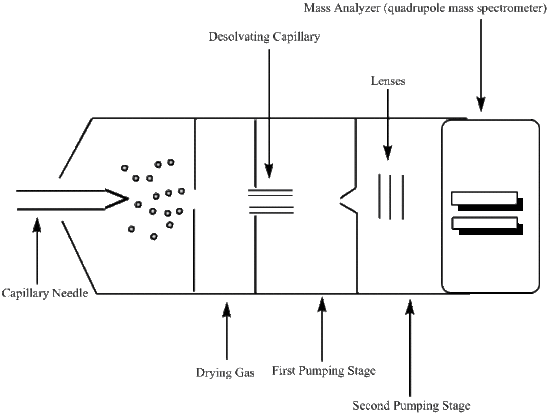
Capillary Needle
The capillary needle is the inlet into the apparatus for the liquid sample. Once in the capillary needle, the liquid sample is nebulized and charged. There is a large amount of pressure being applied to the capillary needle, which in effect nebulizes the liquid sample forming a mist. The stainless steel capillary needle is also surrounded by an electrode that retains a steady voltage of around 4000 volts. This applied voltage will place a charge on the droplets. Therefore, the mist that is ejected from the needle will be comprised of charged molecular ions.
Desolvating Capillary
The molecular ions are oxidized upon entering the desolvating capillary, and a continual voltage is applied to the gas chamber in which this capillary is located. Here the desolvation process begins, through the use of a dry gas or heat, and the desolvation process continues through various pumping stages as the molecular ion travels towards the mass analyzer. An example of a dry gas would be an N2 gas that has been dehydrated. The gas or heat then provides means of evaporation, or desolvation, for the ionized droplets. As the droplets become smaller in size, their electric field densities become more concentrated. The increase in electric field density causes the like charges to repel one another, which induces an increase in surface tension. The point where the droplet can no longer support this increase in surface tension is known as the Rayleigh limit. At this point, the droplet divides into smaller droplets of either positive or negative chrage. This process is refered to as either a coulombic explosion or the ions are described as exiting the droplet through the "Taylor cone". Once the molecular ions have reached the entrance to the mass analyzer, they have been effectively reduced through protonation.
Mass Analyzer
Mass Analyzers (Mass Spectrometry) are used to determine the mass-to-charge ratio (m/z), this ratio is used to differentiate between molecular ions that were formed in the desolvating capillary. In order for a mass-to-charge ratio to be determined, the mass analyzer must be able to separate even the smallest masses. The ability of the analyzer to resolve the mass peaks can be defined with the following equation;
\[R = \dfrac{m}{\Delta m} \]
This equation represents the mass of the first peak (m), divided by the difference between the neighboring peaks \(\Delta m\). The better the resolution, the more useful the data. The mass analyzer must also be able to measure the ion currents produced by the multiply charged particles that are created in this process.
Mass analyzers use electrostatic lenses to direct the beam of molecular ions to the analyzer. A vacuum system is used to maintain a low pressure environment in order to prevent unwanted interactions between the molecular ions and any components that may be present in the atmosphere. These atmospheric components can effect the determined mass-to-charge ratio, so it is best to keep them to a minimum. The mass-to-charge ratio is then used to determine quantitative and qualitative properties of the liquid sample.
The mass analyzer used for electrospray ionization is a quadrupole mass spectrometer. A quadrupole mass spectrometer uses four charged rods, two negatively charged and two positively charged, that have alternating AC and DC currents. The rods are connected to both the positive terminal of the DC voltage and the negative terminal. Each pair of rods contains a negatively charged rod and a positively charged rod. The molecular ions are then sped through the chamber between these pairs of oppositely charged rods making use of a potential difference to do so. To maintain charge, and ultimately be readable by the detector, the molecular ions must travel through the quadrupole chamber without touching any of the four charged rods. If a molecular ion does run into one of the rods it will deem it neutral and undetectable.
Detector
The molecular ions pass through the mass analyzer to the detector. The detector most commonly used in conjunction with the quadrupole mass analyzer is a high energy dynode (HED), which is a electron multiplier with some slight variations. In an HED detector, the electrons are passed through the system at a high voltage and the electrons are measured at the end of the funnel shaped apparatus; otherwise known as the anode. A HED detector differs from the electron multiplier in that it operates at a much higher sensitivity for samples with a large mass than does the electron multiplier detector. Once the analog signal of the mass-to-charge ratio is recorded, it is then converted to a digital signal and a spectrum representing the data run can be analyzed.
References
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis. Sixth Edition, Thomson Brooks/Cole, USA (2007).
- Siuzdak, G. Mass Spectrometry for Biotechnology. Academic Press, Inc., USA (1996).
- Chapman, J.R. Mass Spectrometry of Proteins and Peptides. Methods in Molecular Biology volume 146,Humana Press, Totowa, NJ (2000).
- Snyder, A. P. Biochemical and Biotechnological Applications of Electrospray Ionization Mass Spectrometry. American Chemical Society, Washington, DC (1996).
Problems
Using the above spectrum, calculate the mass of the protein. For p1, shown in spectrum, the m/z is 7501 and for p2 the m/z is 15001. (Hint: Use the above equations, and the charge of p1 for z1)
Contributors and Attributions
- Jennifer Murphy

