Case Study: Battery Types
- Page ID
- 286
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ranging from the very crude to the highly sophisticated, batteries come in a plethora of variety. Batteries in short are electrochemical cells that produce a current of electricity via chemical reactions. More specifically, batteries produce electrical energy from oxidation-reduction reactions. A collection of electrochemical cells wired in series is properly called a battery. A flashlight battery is really a single electrochemical cell, while a car battery is really a battery since it is three electrochemical cells in series.
Introduction
Electrochemical cells have been in use longer than what was once thought. Discovered in Khujut Rabu of modern day Iraq and dating from the Parthian (250 B.C.-A.D. 224) and Sassanid (A.D. 224-600) 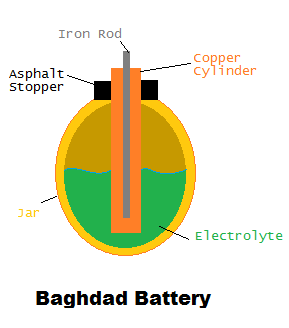 periods, the Baghdad Battery is the first known battery in the world. Consisting of a copper cylinder, an iron rod, an asphalt stopper, and a small earthenware jar, the Baghdad Battery was filled with an unknown electrolytic solution and may have been used for electroplating.
periods, the Baghdad Battery is the first known battery in the world. Consisting of a copper cylinder, an iron rod, an asphalt stopper, and a small earthenware jar, the Baghdad Battery was filled with an unknown electrolytic solution and may have been used for electroplating.
About 2000 years later the Voltaic Pile, a stack of individual cells of zinc and copper disks immersed in sulfuric acid, was created by the Italian Count Volta and effectively replaced the use of the Leyden Jar, an instrument that stored static electricity for future use. Volta's battery is considered the first electrochemical cell and the reaction for which is as follows:
oxidation half-reaction: \(Zn \rightarrow Zn^{2+} + 2e^-\)
reduction half-reaction: \(2H^+ + 2e^- \rightarrow H_2\)
Because zinc is higher in the electrochemical series, the zinc anode reacts with sulfate anions and is oxidized whilst protons are reduced to hydrogen gas. The copper cathode remains unchanged and acts only as electrode for the chemical reaction. Because the Voltaic pile was unafe to use and the cell power diminished over time, it was abandoned.
Electrochemical cells typically consist of an anode (the negative electrode where oxidation occurs), a cathode (the positive electrode where reduction occurs), and an electrolyte (the medium conducting anions and cations within a reaction) all contained within a cell. Electrons flow in a closed circuit from the anode to the cathode. Depending on the configuration of the cell and the electrolyte used, a salt bridge may be necessary to conduct ions from one half cell to another as an electric charge is created when electrons move from electrode to another. The difference created would keep electrons from flowing any further. Because a salt bridge permits the flux of ions, a balance in charge is kept between the half cells whilst keeping them separate.
Types of Batteries
The two main categories of batteries are primary and secondary. Essentially, primary cells are batteries which cannot be recharged while secondary cells are rechargeable. The distinction begs the question as to why primary cells are still in use today, and the reason being is that primary cells have lower self-discharge rates meaning that they can be stored for longer periods of time than rechargeable batteries and maintain nearly the same capacity as before. Reserve and backup batteries present a unique example of this advantage of primary cells. In reserve, or stand-by, batteries components of the battery containing active chemicals are separated until the battery is needed, thus greatly decreasing self-discharge. An excellent example is the Water-Activated Battery. As opposed to inert reserve batteries, backup batteries are already activated and functional but not producing any current until the main power supply fails.
Biobatteries
Devices that generate electric energy via the digestion of carbohydrates, fats, and protiens by enzymes. The most common biobatteries are the lemon or potato battery and the frog or ox-head battery better described as a "muscular pile". In a lemon cell, the energy for the battery is not produced by the lemon but by the metal electrodes. Usually zinc and copper electrodes are inserted into a lemon (the electrolyte being citric acid) and connected by a circuit. The zinc is oxidized in the lemon in order to reach a preferred lower energy level and the electrons discharged provide the energy. Using zinc and copper electrodes, a lemon can produce about 0.9 Volts.
While not technically a biobattery, an Earth battery is comprised of two different electrodes which are either buried underground or immersed in natural bodies of water which tap into Telluric currents to produce electric energy.
Dry-Cell Batteries
During the 1860s, a French man named George Lelanche developed the Lelanche cell also known today as the dry-cell battery. A dry-cell battery is a battery with a paste electrolyte (as opposed to a wet-cell battery with a liquid electrolyte) in the the middle of its cylinder and attached are metal electrodes. A dry-cell battery is a primary cell that cannot be reused. In order to function, each dry-cell battery has a cathode and an anode. Some examples of dry-cell batteries used in everyday objects today are remote controls, clocks, and calculators.

Figure: Modern dry cell batteries. from Wikipedia
Types of dry-cell batteries are zinc-carbon batteries, alkaline-cell batteries, and mercury batteries. Before zinc-carbon batteries were used, mercury batteries were the main resource. It was not until mercury was known to become harmful that zinc-carbon batteries replaced it. Batteries may produce the following potential problems or hazards:
- Pollute the lakes and streams as the metals vaporize into the air when burned.
- Contribute to heavy metals that potentially may leach from solid waste landfills.
- Expose the environment and water to lead and acid.
- Contain strong corrosive acids.
- May cause burns or danger to eyes and skin.
Dry-cell batteries are the most common battery type used today. Essentially, the battery is comprised of a metal electrode (or graphite rod) surrounded by a moist electrolyte paste that is enclosed in a metal cylinder. 1.5 volts is the most commonly used voltage for dry-cell batteries. The sizes of dry-cell batteries vary, however, it does not change the voltage of the battery.
Zinc-carbon cells
Zinc-carbon cells were the first really portable energy source. These cells have a short lifetime and the zinc casings become porous as the zinc is converted to zinc chloride. The substances in the cell that leak out are corrosive to metal and can terminally destroy electronic equipment or flashlights. Zinc-carbon cells produce 1.5 volts.
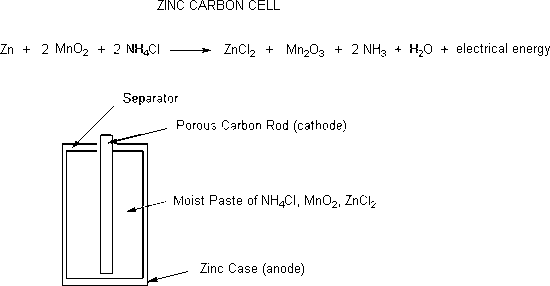
For a dry-cell battery to operate, oxidation will occur from the zinc anode and reduction will take place in the cathode. The most common type of cathode is a carbon graphite. Once reactants have been turned into products, the dry-cell battery will work to produce electricity. For example, in a dry-cell battery, once \(Zn^{2+}\) has been oxidized to react with \(NH_3\), it will produce chloride salt to insure that too much \(NH_3\) will not block the current of the cathode.
\[Zn^{2+}_{(aq)} + 2NH_{3\;(g)} + 2Cl^-_{(aq)} \rightarrow [Zn(NH_3)_2]Cl_{2\; (s)} \nonumber \]
How does the reaction work? While the zinc anode is being oxidized, it is producing electrons that will be captured by reducing Maganese from an oxidation state of +4 to a +3.
- Reduction of Maganese: \(2MnO_{2\;(s)} + H_2O_{(l)} + 2e^- \rightarrow Mn_2O_{2\; (s)} + 2OH^-_{ (aq)}\)
The electrons produced by Zinc will then connect to the cathode to produce it's product.
- Oxidization of Zinc: \(Zn_{(s)} \rightarrow Zn^{2+}_{(aq)} +2e^-\)
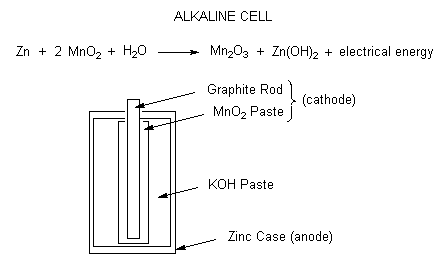
Alkaline cells
Recently, the most popular dry-cell battery to be used has been the alkaline-cell battery. In the zinc-carbon battery shown above, the zinc is not easily dissolved in basic solutions. Though it is fairly cheap to construct a zinc-carbon battery, the alkaline-cell battery is favored because it can last much longer. Instead of using \(NH_4Cl\) as an electrolyte, the alkaline-cell battery will use \(NaOH\) or \(KOH\) instead. The reaction will occur the same where zinc is oxidized and it will react with \(OH^-\) instead.
\[Zn^{2+}_{(aq)} + 2OH^-_{(aq)} \rightarrow Zn(OH)_{2\; (s)} \nonumber \]
Once the chemicals in the dry-cell battery can no longer react together, the dry-cell battery is dead and cannot be recharged. Alkaline electrochemical cells have a much longer lifetime but the zinc case still becomes porous as the cell is discharged and the substances inside the cell are still corrosive. Alkaline cells produce 1.54 volts.
Mercury cells
Mercury batteries are small, circular metal batteries that were used in watches. Mercury cells offer a long lifetime in a small size but the mercury produced as the cell discharges is very toxic. This mercury is released into the atmosphere if the cells are incinerated in the trash. About 90% of the 1.4 million pounds of mercury in our garbage comes from mercury cells. Mercury cells only produce 1.3 Volts.
\[HgO + Zn + H_2O \rightarrow Hg + Zn(OH)_2 \nonumber \]
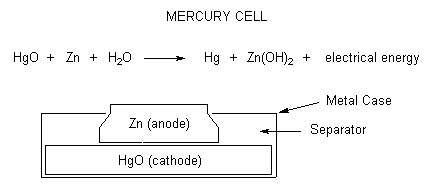
Mercury batteries utilize either pure mercuric oxide or a mix of mercuric oxide with manganese dioxide as the cathode. The anode is made with zinc and is separated from the cathode with a piece of paper or other porous substance that has been soaked in the electrolyte (which is generally either sodium or potassium oxide).
In the past, these batteries were widely used because of their long shelf life of about 10 years, and also because of their stable, steady voltage output. Also, they had the highest capacity per size. They were popular for use in button-type battery applications, such as watches or hearing aids. However, the environmental impact for the amount of mercury present in the batteries became an issue, and the mercury batteries were discontinued from public sale.
lead-acid batteries
The lead-acid battery used in cars and trucks consists of six electrochemical cells joined in series. Each cell in a lead-acid battery produces 2 volts. The electrodes are composed of lead and are immersed in sulfuric acid. The negative electrodes are spongy lead metal and the positive electrodes are lead impregnated with lead oxide. As the battery is discharged, metallic lead is oxidized to lead sulfate at the negative electrodes and lead oxide is reduced to lead sulfate at the positive electrodes. When a lead-acid battery is recharged by an alternator, electrons are forced to flow in the opposite direction which reverses the reaction.
\[Pb + PbO_2 + 2 H_2SO_4 \rightarrow 2 PbSO_4 + 2 H_2O \nonumber \]
Nickel-cadmium cells
Nickel-cadmium cells can also be regenerated by reversing the flow of the electrons in a battery charger. The cadmium is oxidized in these cells to cadmium hydroxide and the nickel is reduced. Nickel-cadmium cells generate 1.46 Volts.
\[Cd + NiO_2 + 2 H_2O \rightarrow Cd(OH)_2 + Ni(OH)_2 \nonumber \]
A Nickel-metal Hyride battery is a secondary cell very similar to the nickel-cadmium cell except that it uses a hydrogen-absorbing alloy in place of cadmium. The Nickel-metal Hyride battery has 2-3 times the capacity of a nickel-cadmium cell.
Questions
- Why are lead-acid batteries used in cars?
- What is the specific, more scientific name for a battery?
- how do batteries generally work?
- What kind of reaction occurs at the Cathode?
- What kind of reaction occurs at the anode?
- What type of battery can be recharged? Which cannot?
- If mercury batteries are so long lasting and efficient, then why are they not used anymore?
- What is the purpose of a salt bridge?
Answers
- Lead acid batteries produce two volts each and last a relatively long time. This type of battery is good for cars because of its ability to be recharged by the alternator. The batteries are connected in a series to produce the necessary voltage. They are larger than most other batteries, but work well for large energy-consuming machinery such as vehicles.
- Batteries are actually called electrochemical cells.
- Batteries work through a series of oxidation-reduction reactions that produces a waste product and a known amount of energy.
- Reduction reaction.
- Oxidation reaction.
- Secondary batteries can be recharged. Primary batteries cannot be recharged because the reaction is not reversible.
- Mercury batteries are often disposed incorrectly, which causes large amounts mercury to seep into the environment. Most or the mercury present in our environment today is the result of improper mercury battery disposal.
- Salt bridges conduct ions from one half cell to another to balance changing charges that could cause a halt to the flow of electrons in an electrochemical cell.
Contributors and Attributions
- Richard Banks (Boise State University)
- Erica Chen (UCD)

