Sample Preparation
- Page ID
- 152825
At the end of this assignment students will be able to:
- Define components and operation of common sample preparation methods.
- Assess the benefits and limitations of different extraction methods.
- Validate extraction efficiency.
- Determine an appropriate extraction and preconcentration method for an analysis.
In the last module, you examined a process for collecting samples from Lake Nakuru for analysis. In many analytical measurements, the samples cannot be analyzed directly without some form of pretreatment. That will also be the case with the samples from Lake Nakuru. The aqueous samples you have collected cannot be injected directly into a gas chromatograph-mass spectrometer (GC-MS), and must go through a pretreatment process prior to analysis.
Purpose
The purpose of this assignment is to address questions related to the sample preparation portion of EPA method 525-2 for measurement of organochlorine pesticides by GC-MS.
Why do samples often need pre-treatment before analysis?
There are three key issues that often arise that necessitate pre-treatment of a sample prior to analysis: (1) the sample is in the wrong physical state for the analysis method (e.g., the method requires a liquid but you have a solid sample), (2) the sample has interfering matrix components that may give either a false positive or negative reading in the measurement, and (3) the sample has too low an analyte concentration to be detected by the instrument.
Consider the aqueous samples from Lake Nakuru, and discuss the following questions.
Q1. Is the sample in the wrong physical state for the analysis method?
Q2. Does the sample have interfering matrix components that may give either a false positive or negative reading in the measurement?
Q3. Does the sample have too low an analyte concentration to be detected?
With the answer to the above questions, it is apparent that the water samples from Lake Nakuru will need to be pretreated prior to the GC-MS analysis.
Q4. Can you think of a procedure to remove the organochlorine pesticides from water?
In other laboratories, for example in organic chemistry lab, you may have performed a liquid-liquid extraction to isolate a product or remove a contaminant. It is possible to use a liquid-liquid extraction with a water-immiscible organic solvent to remove organochlorine pesticides from water. A more common form of sample pretreatment used today involves a liquid-solid extraction. In this procedure, the liquid phase (in our case, the water sample) is passed through a cartridge or disk that is packed with a solid material. The goal is to have the organochlorine pesticides adsorb to the surface of the solid while the water passes through. You are probably familiar with water purification filters that can be attached to a tap to provide better drinking water. These operate under the same principle as they are solid extraction cartridges that are designed remove components of the water that are not desirable to drink.
Take a look at the following section (7.2) from the EPA procedure:
“Liquid-Solid Extraction (LSE) Cartridges -- Cartridges are inert non-leaching plastic, for example polypropylene, or glass, and must not contain plasticizers, such as phthalate esters or adipates, that leach into the ethyl acetate and methylene chloride eluant. The cartridges are packed with about 1 g of silica, or other inert inorganic support, whose surface is modified by chemically bonded octadecyl (C18) groups. The packing must have a narrow size distribution and must not leach organic compounds into the eluting solvent. One liter of water should pass through the cartridge in about two hours with the assistance of a slight vacuum... The extraction disks contain octadecyl bonded silica uniformly enmeshed in an inert matrix. The disks used to generate the data in this method were 47 mm in diameter and 0.5 mm in thickness. Other disk sizes are acceptable and larger disks may be used for special problems or when sample compositing is carried out. As with cartridges, the disks should not contain any organic compounds, either from the matrix or the bonded silica, which will leach into the ethyl acetate and methylene chloride eluant. One L of reagent water should pass through a disc in five to 20 min using a slight vacuum.”
The procedure says that “one liter of water should pass through the cartridge in about two hours with the assistance of a slight vacuum.”
Q5. Why is this procedure done over two hours instead of 10 minutes?
The procedure also specifies the use of an octadecyl cartridge.
Q6. Why is octadecyl chosen as an extraction stationary phase for the study of pesticides? What would happen if bare silica was used as an extraction phase?
You now have the pesticides adsorbed onto the solid phase cartridge, but they are still not in a form suitable for analysis by GC-MS. The elution step involves the use of a liquid solvent to desorb the organochlorine pesticides from the solid phase.
Q7. Discuss the following aspects of the solvent you would choose to elute the organochlorine pesticides.
- Polarity
- Boiling point
- Volume
The elution step you have devised in response to this last question will likely result in an organic extract that contains some water. It is not desirable to have water in the samples that will be injected into the GC-MS instrument because water can damage the column.
Q8. How would you remove any residual water in the organic phase?
For analytes present at low concentration, the volume of the organic extract obtained in the elution step is still too large and the sample too dilute for analysis.
Q9. How would you reduce the volume of the organic extract that contains your pesticide?
Q10. What concerns might you have in the solvent reduction step?
Below is the section of the EPA method about the concentration of the extract. The process of desorption of the organochlorine pesticides from the solid cartridge into an appropriate solvent at an appropriate volume is referred to as sample reconstitution. Sometimes the organic compounds are removed with a solvent that is especially suitable for the desorption step, but this solvent is then evaporated and the compounds taken up in another solvent more suitable for the analysis.
For example, see Section 11.2.9. “While gently heating the extract in a water bath or a heating block, concentrate to between 0.5 mL and 1 mL under a gentle stream of nitrogen. Do not concentrate the extract to less than 0.5 mL, since this will result in losses of analytes. Make any volume adjustments with ethyl acetate. It is recommended that an aliquot of the recovery standard be added to the concentrated extract to check the recovery of the internal standards (see Section 7.12).”
Usually, before doing such a sample pretreatment, the analyst has done calculations to ensure that the final solutions will have sufficient concentrations of the analyte to be quantified by the instrument. Let’s assume that the lake water has an average concentration of DDT of 150 ng/L (ppt). The limit of detection (LOD) for the GC-MS procedure is 0.083 μg/L (ppb).
Q11. What would be the final extraction concentration be if you plan to use EPA Method 525.2? Can you detect this level in the lake water samples?
Q12. What does it mean to determine the precision of the pesticide analysis? What does it mean to determine the percent recovery of the pesticide?
Q13. Suppose a solid-phase extraction procedure provided a low percent recovery. What could you change in an attempt to raise this value?
The following table is real data obtained from the EPA method.
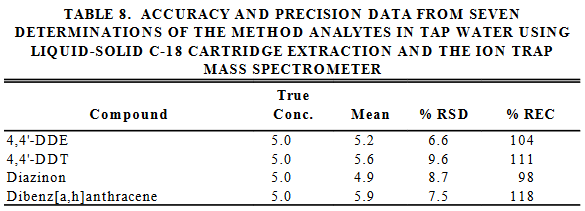
Q14. Is the solid used in the cartridge effective for removing the pesticides from water?
Q15. How is it possible to get a percent recovery of over 100?
In addition to reconstitution, additional steps are often needed to preserve or prepare samples for analysis. The EPA Method 525.5 specifies that the water samples acquired should be specifically treated to preserve the pesticides:
“8.2 Sample Dechlorination and Preservation -- All samples should be iced or refrigerated at 4 °C and kept in the dark from the time of collection until extraction. Residual chlorine should be reduced at the sampling site by addition of 40-50 mg of sodium sulfite (this may be added as a solid with stirring or shaking until dissolved) to each water sample. It is very important that the sample be dechlorinated prior to adding acid to lower the pH of the sample. Adding sodium sulfite and HCl to the sample bottles prior to shipping to the sampling site is not permitted. Hydrochloric acid should be used at the sampling site to retard the microbiological degradation of some analytes in water. The sample pH is adjusted to <2 with 6 N hydrochloric acid. This is the same pH used in the extraction, and is required to support the recovery of acidic compounds like pentachlorophenol.
8.3 Holding Time -- Results of the time/storage study of all method analytes showed that all but six compounds are stable for 14 days in water samples when the samples are dechlorinated, preserved, and stored as described in Section 8.2. Therefore, samples must be extracted within 14 days. If the following analytes are to be determined, the samples cannot be held for 14 days but must be extracted immediately after collection and preservation: carboxin, diazinon, disulfoton, disulfoton sulfoxide, fenamiphos, and terbufos. Sample extracts may be stored at 4 °C for up to 30 days after sample extraction.”
Q16. From the chemistry described in sections 8.2 and 8.3 of the EPA method, what might be the cause of pesticide degradation before extraction?
Standards, Spikes and Surrogates
Note: A discussion of standards, spikes and surrogates will also be covered in the method validation section. This section will highlight their roles in sample preparation.
Take a look at the following excerpt from the EPA Method.
“7.8 Fortification Solution of Internal Standards and Surrogates -- Prepare an internal standard solution of acenaphthene-d10, phenanthrene-d10, and chrysene-d12, in methanol, ethyl acetate, or acetone at a concentration of 500 μg/mL of each. This solution is used in the preparation of the calibration solutions. Dilute a portion of this solution by 10 to a concentration of 50 μg/mL and use this solution to fortify the actual water samples (see Section 11.1.3 and Section 11.2.3). Similarly, prepare both surrogate compound solutions (500 μg/mL for calibration, 50 μg/mL for fortification). Surrogate compounds used in developing this method are 1,3-dimethyl-2-nitrobenzene, perylene-d12, and triphenylphosphate. Other surrogates, for example pyrene-d10 may be used in this solution as needed (a 100 μL aliquot of this 50 μg/mL solution added to 1 L of water gives a concentration of 5 μg/L of each internal standard or surrogate). Store these solutions in an amber vial at 4 °C or less. These two solutions may be combined or made as a single solution.”
Q17. What is a surrogate compound?
Q18. What assumptions must be made when choosing a surrogate?
Q19. When is the surrogate added during the sample preparation (i.e. before extraction or after) and why is it added then?
Take a look at the following tables taken from the EPA Method 525.2.
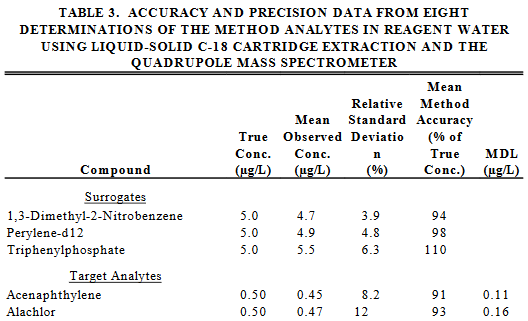
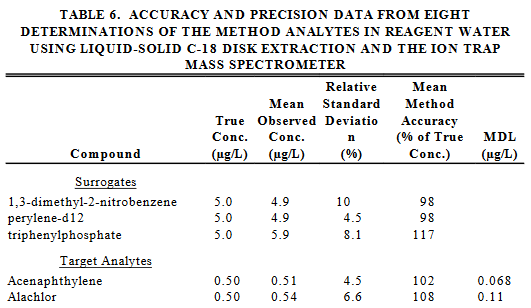
Q20. What could be responsible for the different percentages of true concentration for the surrogates in the two tables?
Q21. What is an internal standard? Why is it used? At what point during the sample preparation process are internal standards added?
Q22. Why spike samples? Do you spike them before or after the sample preparation?
Helpful Resources
Harvey, D. Chapter 7, Collecting and Preparing Samples.


